We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
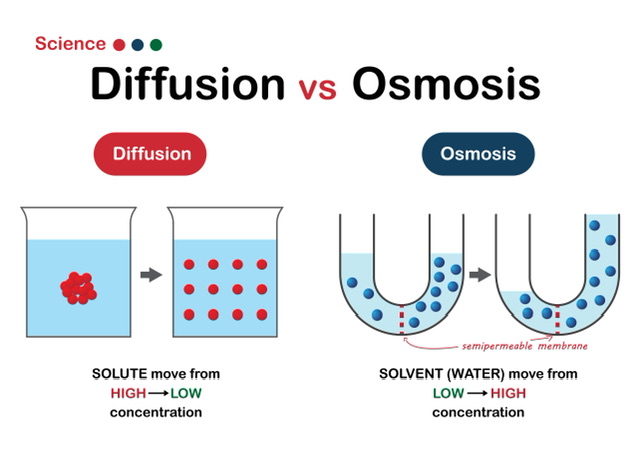
It's common to wonder how to tell the two processes apart. Hence, we need to separate these two processes in order to get a handle on this. The major difference between osmosis and diffusion is that in the former, solvents move, while in the latter, molecules move. That's why you may call them both "passive" modes of travel. What's more, it's an intrinsic bodily process that promotes molecular mobility without expending any extra energy. There is a possibility that this shift is from higher to lower concentration, or vice versa.
Osmosis is the movement of solute molecules over a semipermeable membrane. Moreover, it happens when a diluted solution is transformed into a concentrated one. Balance on both sides of the membrane is also helped along by this. Therefore the movement of H2O molecules is also called the solvent. Also, H2O (water) molecules diffuse from high to low concentrations. The fact that these particles can diffuse through a semipermeable membrane is the most critical. Hence, osmosis is not the same as other forms of diffusion. It's important for transporting nutrients across the body and flushing out metabolic waste.
Some common examples of osmosis are:
Since they are constructed from a material that allows water but not larger molecules to flow through, osmosis is used in water treatment filters.
The hormone ADH triggers the kidney collecting tubule to reabsorb water (H2O).
The process by which fishes expel the most liquid with the least amount of salt loss, which is achieved by the excretion of very diluted pee.
People lose water (H2O) through a process called osmosis when they sweat.
It's the process of moving solids, liquids, and gases from high- to low-density areas. The vast number of particles in motion at any given time constitutes free energy and is responsible for this motion. Hence, they achieve a state of free energy and particle diffusion equilibrium when they move to areas of lower concentration. In addition, there is no partially permeable barrier here. For animals, oxygen is also vital for producing energy and exchanging gases during breathing. It's not just during photosynthesis that plants need it, but also throughout the process of transpiration.
Some common examples of diffusion are:
Transportation of oxygen to and from the pulmonary alveoli.
The axon membrane allows the passage of sodium (Na) and potassium (K) ions, which are part of nerve impulses.
When two metals contact via their faces and produce a diffusing pair, the temperature drops below the melting point, confirming the composition: the nickel (Ni) atoms have melted towards the copper (Cu).
Coffee's temperature and color shift when cold milk is poured in large amounts.
Diffusion is an integral part of many biological systems, including those that regulate and supply the body with substances necessary for survival. Gas exchange is one of the mechanisms that occurs. Oxygen is taken in for the metabolic process of respiration, while carbon dioxide is exhaled as a waste product. The many alveoli in a mammal's lungs are the site of this interaction.
Osmosis serves to keep the internal and external surroundings in equilibrium. In the case of a plant, water can osmotically go from the soil to the root hairs. It moves from the mud to a lower concentration area because of the mud's higher water content (i.e., plant)
Title: Diffusion and Osmosis procedure
The similarities between both the processes are:
In both diffusion and osmosis, particles move from a high concentration to a low concentration zone.
The end result of both procedures is a stable equilibrium between two solutions.
Both diffusion and osmosis are examples of passive transport systems; they may move their cargo along without expending any additional energy.
By passing over a semipermeable membrane, substances can be diffused to equalize concentrations of two separate substances, a process known as osmosis. Depending on the concentration of a solute, like salt, water will either flow into or out of a cell on its own. The net migration of particles from a location of high concentration to a region of low concentration can be explained by the physical phenomena of diffusion. Its constituents can exist in various states, including solid, liquid, and gas. These two modes of transportation are considered passive since they do not produce any sort of energy themselves.
Raisins are dry with little water within; But when placed in water, osmosis occurs, and water passes through the cell wall and cell membrane of the raisins, causing them to expand.
Osmotic pressure is a colligative property. As the amount of the substance increases, the osmotic pressure increases and the rate of osmosis increases as well.
Plant cells lose water by osmosis when not present in a favorable environment or after ageing. The components of the cell shrivel or contract away from the cell wall. This is referred to as plasmolysis.
