We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
Atoms and molecules are essential for life. All living things are composed of molecules, and the chemical reactions in living organisms are driven by the interactions between atoms and molecules. For example, photosynthesis, which is the process by which plants convert sunlight into energy, is driven by the interactions between molecules of carbon dioxide and water.
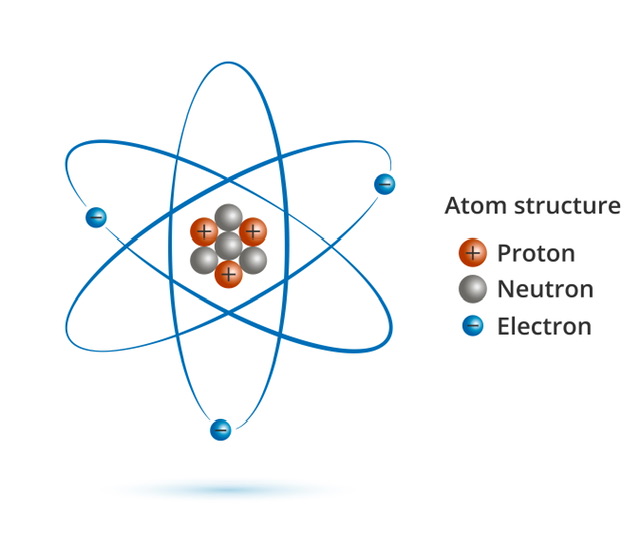
The smallest units of matter are atoms and molecules. Atoms, which consist of protons, neutrons, and electrons, are the smallest possible particles of an element. All chemical compounds begin with molecules, which consist of two or more atoms bound together.
The atom is what makes an element unique; it cannot be broken down chemically. The nucleus of a typical atom is composed of neutral protons and neutrons, while electrons, which carry negative charges, circle the nucleus.
The size of an atom depends on its number of protons, neutrons, and electrons, as well as the existence or absence of electrons. A typical atom is around 100 picometers in size or 1/10 billionth of a metre.
Title: Atom
The term "modern atomic theory" describes the most up-to-date understanding of how atoms work. The contemporary atomic theory provides the best account to yet of the appearance and behaviour of atoms, according to those who study them.
Quanta electrons are attracted to the nucleus' positive charge and cluster around it due to electrostatic repulsion. Most simplistic diagrams of atoms show electrons revolving around the nucleus like planets around the sun, represented by spherical or particle representations. The reality is that the nature of electrons is not depicted accurately in this image. The modern atomic model instead characterises electrons as clouds, waves, probability functions, and the indeterminate.
Elements are chemistry's most fundamental building blocks since they cannot be broken down any further. Certain atoms of each element cannot be found in any other element.
Yet, atoms are capable of disintegrating into even smaller particles. Atoms of each element contain the same number of protons located in the nucleus. The nucleus also contains neutrons, however the precise quantity may vary amongst isotopes of the same atomic type.
Atoms in a molecule is held in place by chemical bonds, creating a precise structure. A molecule, such as O2, might consist of two or more of the same element's atoms or of atoms from various elements. The properties of a chemical are determined by the arrangements of its atoms within its molecule. All of the atoms in the molecule have masses that are close to or equal to the molecular weight.
There are generally three types of bonds in an atom:
Ionic bonds: Ionic bonds are formed when valence electrons are shared across atoms to complete their outermost shells. NaCl, or table salt, is an ionically bonded molecule because sodium gives up its outermost electron so that chlorine can do the same.
Covalent Bonds: Covalent bonds form when the outermost shell electrons of two or more different atoms are shared. Polymers are an example of materials that use covalent bonding. Polymers consist of long strands of hydrogen and carbon atoms bonded together by covalent bonds.
Metallic Bond: A metallic bond is formed when the electrons in the outermost shell are not paired with any particular atom or ion but instead exist as a "cloud" of electrons surrounding the ion centres. Magnesium, Sodium, and Aluminium are all examples of elements that form metallic bonds. Metallic bonding results in the metal's characteristic properties or attributes, such as its malleability, thermal and electrical conductivity, and luster
An ionic bond forms when the valence electrons of one atom are transferred to those of another. An atom becomes a negatively charged ion (anion) if it gains an electron and a positively charged ion (cation) if it loses one ( cation). The molecule has a neutral charge since the positive and negative ions cancel each other out.
It is generally agreed upon in the scientific community that the smallest unit of an element that may or cannot exist freely is called an atom. In contrast, the smallest unit of a compound is a molecule, which consists of a collection of atoms bound together by chemical forces. A state of freedom for an atom is also possible. molecules exist in a free state, however. In addition, the nucleus of an atom contains protons and neutrons, while the electrons orbit around the nucleus. Yet, a molecule is made up of two or more atoms that are chemically bound together and might be the same or distinct from one another.
A chemical bond is an attractive force between two atoms or molecules that allows them to form a chemical compound. The types of chemical bonds include covalent bonds, ionic bonds, and hydrogen bonds.
Democritus, a Greek philosopher, proposed the concept of the atom around 450 B.C.
In spite of this, for almost two thousand years nobody paid much attention to the concept at all. John Dalton re-introduced the atom in 1800. He established the existence of atoms and pioneered the field of atomic theory.
Isotopes are the extended families of elements. In an isotope, the number of neutrons can vary, but the number of protons remains constant among all members of the element's family. In the Periodic Table, an element's atomic number corresponds to its number of protons.
