We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
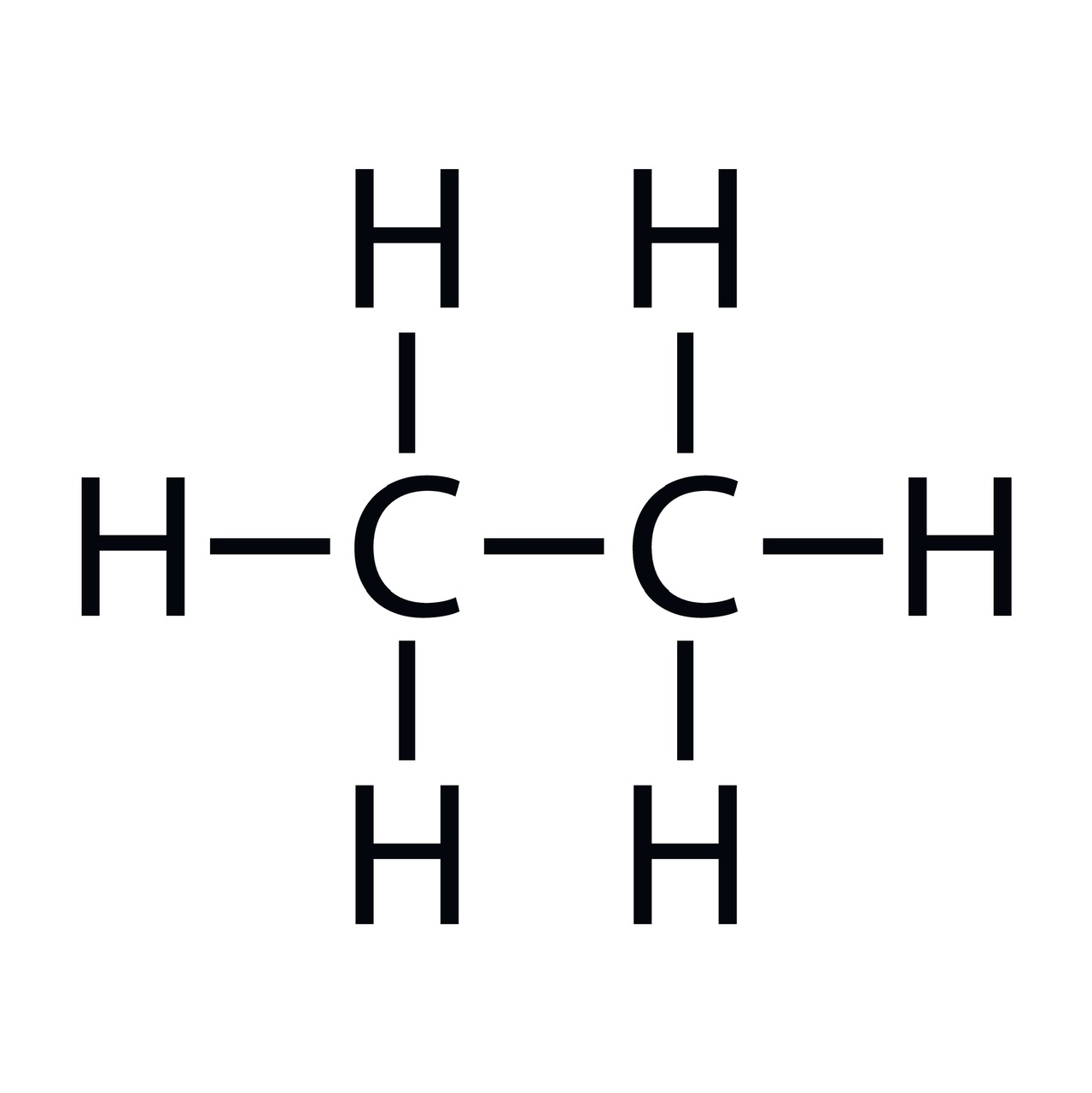
In organic chemistry, aliphatic hydrocarbons and aromatic hydrocarbons are two distinct classes of hydrocarbons. Chemical compounds with only one connection between their atoms are called aliphatic compounds. In carbon compounds, the atoms are bound together by the exchange of valence electrons. Covalent bonds hold them together, to put it another way. Aliphatic chemicals can have linear, branching, or cyclic molecular structures.
The Greek language is the source of the word aliphatic. Long hydrocarbon chains make up fats and oils. Hence this term was employed to characterize these organic compounds. Hydrocarbon chains may be recognized by their linearity; the chain is open, and the carbon atoms are connected in a snakelike pattern.
When classifying compounds as aliphatic or non-aliphatic, the order of the carbon atoms is not significant. Aliphatic compounds may be easily identified by their single- or double-chain carbon-carbon bond structure. Aliphatic compounds include double or triple carbon bonds as well. A functional group can be present in an aliphatic chemical. These functional groupings often include non-carbon and non-hydrogen atoms.
The chemical characteristics of aliphatic molecules are a direct result of the functional groups to which they are attached. What follows is a bulleted list describing the common characteristics of aliphatic compounds. These characteristics aren't restricted to any one class of aliphatic compounds.
The boiling and melting points rise as the molecular weight increases of a compound.
Unlike polar hydrocarbons, aliphatic hydrocarbons do not dissolve in water.
As an organic molecule branches out, the boiling weight goes up. The boiling point of a same-sized linear molecule would be lower than that of an equivalent-sized branched molecule.
All aliphatic chemicals, but notably hydrocarbons, are very combustible. As a result, methane, ethane, and butane molecules are employed as a source of energy in many different contexts. Specifically in many home and car appliances.
A common characteristic of aliphatic chemicals is a strong, gasoline-like odor.
Sometimes, aliphatic compounds containing polar functional groups are miscible with water. This is only in the absence of a preponderantly hydrophobic organic component.
Linear aliphatic compounds: Ethane, pentane, hexane, ethylene, etc
Ethane
Branched aliphatic compounds: isopropane, methylhexane, etc.
Cyclic aliphatic compounds: cyclohexane, cyclopropane, cyclopentane, etc
What is an aromatic compound?
Aromatic compounds are a class of unsaturated organic molecules characterized by at least one planar ring of atoms and alternating double and single bonds within that ring.
Every single carbon forms a double bond with its surrounding carbon in the cyclic structure of these molecules. Conjugated bonds are those that feature consecutive single and double bonds. Resonance, or the movement of electrons from one atom to another, delocalized the density of electrons in conjugated rings. Because of the network of electrons, the bonds in aromatic compounds delocalize on both sides of the ring, resulting in a flat geometrical shape.
Characteristics of Aromatic Compounds
The main characteristics of aromatic compounds are:
At least one carbon ring is present
The ring should show complete conjugation
The coplanar ring structure results from the resonant passage of electrons along the length of the ring in both directions.
All of the ring's carbon atoms must be sp2 hybridized. The ring must be completely flat. An sp3 hybridized carbon atom would have a tetrahedral shape.
Huckel's Rule classifies molecules as aromatic, anti-aromatic, or non-aromatic according to the number of electrons and the ring structure.
According to this rule, the number of pi electrons (N) should follow the following equation:
N= 4n + 2
Where, n = the number of carbon atoms.
A cyclic resonating compound that follows Huckel’s rule is said to be aromatic. If the compound does not follow huckle’s rule but shows resonance, then it is called non-aromatic.
Well-known examples of aromatic compounds are benzene and toluene. There is just one aromatic ring in benzene. Simply put, toluene is a methylated form of benzene. The simplest aromatic compounds may be derived from coal and petroleum. They are also present in oil shale. Aromatic compounds emit a pleasing odor. Whereas benzene and toluene have pleasant aromas, aniline has the distinct odor of decaying fish. This chemical, 4,4′-methylenedianiline, is an aromatic compound with no discernible smell (MDA). Aromatic compounds are produced as a byproduct of geological eruptions, wildfires, and the combustion of fuels.
Aliphatic hydrocarbons and aromatic hydrocarbons are two types of hydrocarbons that play different roles in organic chemistry. Aliphatic compounds are chemical compounds that have only a single bond between their atoms. The atoms in carbon compounds are held together by transferring valence electrons. As an alternative way of putting it, covalent bonds keep things together. The molecular architectures of aliphatic compounds can be either linear, branched, or cyclic. Aromatic compounds follow Huckle’s rule. They are characterized by a certain spell that gives them their name.
1. What causes aromatic compounds to catch fire easily?
Any aromatic hydrocarbons may be burned with sufficient heat. Because of their high vapor pressures and low flash points, benzene, and substituted benzenes—the lightest members of this class—pose a risk of vapor explosions.
2. Why do aromatic compounds not show aromatic reactions?
Despite having many double bonds, aromatic compounds are unable to participate in addition reactions. Complete -electron delocalization leads to very stable ring structures, which account for their low reactivity towards addition processes (resonance).
3. Do all molecules in the aliphatic class repel water?
Nonpolar and hydrophobic R groups characterize aliphatic compounds. There is a correlation between the length of a hydrocarbon chain and its hydrophobicity. Although these amino acids are more at home inside proteins, alanine, and glycine are ambivalent and can exist either inside or outside the protein molecule.
