We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
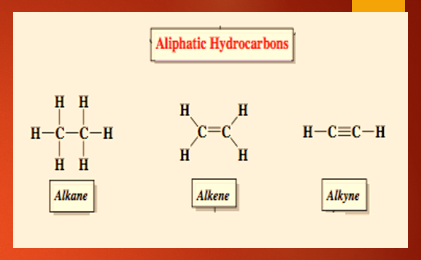
Aliphatic hydrocarbon is the other class of hydrocarbon compounds apart from an aromatic hydrocarbon containing usually two atoms i.e, Hydrogen and Carbon joined together in a chain form, branched form or non-aromatic ring form. In the parent chain or the main chain of hydrocarbon C-C bonding is present and the hydrogen is attached to the carbon.
The first and simplest example of aliphatic hydrocarbon is methane represented by the formula CH4. Apart from hydrogen, different atoms like oxygen, chlorine, sulphur and nitrogen can be bound with carbon to form different chemical compounds.
List of aliphatic hydrocarbons :
Hydrocarbons are compounds mainly comprising two atoms i.e, carbon and hydrogen. There is two-division of hydrocarbons (i) Aliphatic hydrocarbons and (ii) Aromatic hydrocarbons. In aliphatic hydrocarbons, the atoms are arranged in chains in, branched form or in cyclic form.
Based on the type of bonds present between the carbon atoms, the aliphatic hydrocarbons are distributed into (i) saturated hydrocarbons and (ii) unsaturated hydrocarbons. Saturated hydrocarbons are the ones where the carbon-carbon atom is bonded with a single bond. Example: Methane, propane Unsaturated hydrocarbons are compounds where the carbon-carbon atom is bonded with a double bond or triple bond. Example: Ethene, propene, ethyne, propyne. The saturated and unsaturated aliphatic hydrocarbons dissimilarities are shown below.
An increase in the number of atoms present in a chemical compound results in stronger intermolecular attractions. This causes changes in the physical properties. The melting point and the boiling point changes as the carbon and hydrogen atoms differ. Alkanes are highly stable compounds but on interacting with heat/light the carbon-hydrogen bond or carbon-carbon breaks down.
An example is combustion reaction:
Alkanes burn on coming in contact with oxygen resulting in an exothermic reaction where the products formed are carbon dioxide CO2 and H2O. For this reason, alkanes are used as excellent fuels.
Butane is the gas used in stoves and lighter. Gasoline is a mixture of alkanes having up to nine carbon atoms which are added to fuel to improve its performance.
Due to the non-polar nature of hydrocarbons, it is insoluble in water and different from other polar solvent. Hydrocarbons show dissolution in a non-polar solvent like benzene. Hydrocarbons are usually denser than water that's why they float on the water surface. Ethylene is a neutral colourless gas with a sweet odour. Ethene is used in the manufacturing of polyethylene, petrochemical, and polymers. Ethene is lighter in weight than air. Ethylene is formed industrially by the method of cracking.
Halogens react with alkenes compounds and alkynes but both the processes somehow differ. This reaction is known as an additional reaction.
The example is shown below.
Alkanes: Methane, ethane, propane etc.
Alkenes: Ehene, propene, butene
Alkynes: Ethyne, butyne
Cycloalkanes: Cyclopropane
Cycloalkenes: Cyclohexene
Cycloalkynes: Cyclooctyne
Pressurized fluid extraction abbreviated as PFE is the method used for the extraction of aliphatic compounds. Here organic solvents are used. Aliphatic hydrocarbons are extracted from a solid environmental sample like a dead matter of plants and animals. Hydrocarbons are not used as a solvent but they are often used as diluents for different solvents.
Most of the aliphatic hydrocarbons are toxic in nature and vastly affect the body. The aliphatic hydrocarbons are misused by the industries and also used in households. Inhalation of volatile hydrocarbons causes many health issues. It mainly targets three organs: the central nervous system, the lungs and the heart. It leads to gastrointestinal symptoms which include vomiting, diarrhoea, and abdominal pain.
Crude oil and natural gas are the main sources of hydrocarbons. Petroleum is extracted from the ground that contains numerous straight-chain alkanes, and aromatic hydrocarbons. The aromatic hydrocarbon has four to numerous hundred carbon atoms.
The compounds formed mainly of hydrogen and carbon are known as hydrocarbons which are further classified into aliphatic hydrocarbons and aromatic hydrocarbons. Hydrocarbons are obtained naturally from crude oils and also natural gas. Aliphatic hydrocarbons on the basis of bonds are divided into unsaturated hydrocarbons and saturated hydrocarbons. Saturated hydrocarbons include alkanes with C-C single bond and unsaturated hydrocarbons include alkenes and alkynes with C-C double and triple bond respectively.
1. Write one difference between aromatic hydrocarbon and aliphatic hydrocarbon?
Ans: Aliphatic hydrocarbons are in the form of branched, ring and cyclic structure whereas aromatic hydrocarbons are in the form of ring structure with delocalized pi orbital.
2. Name the source for Terpenes?
Ans: Terpenes are the plant hydrocarbon present in pine oil, camphor, turpentine.
3. What are crude oil and natural gas?
Ans: Crude oil and natural gas are fossil fuels. They are formed by the decomposition of dead organisms.
