We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
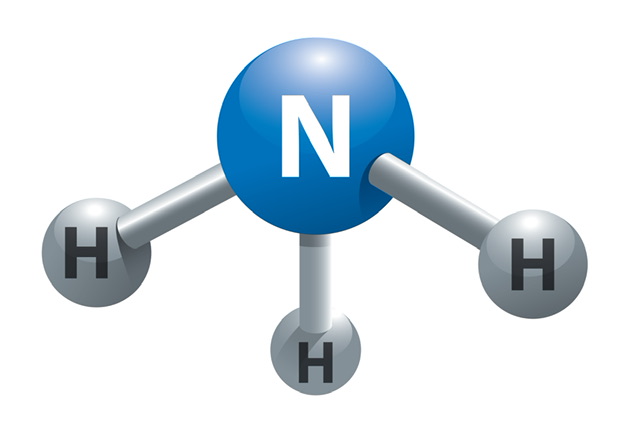
Ammonia, or NH3, is a gas made up of hydrogen and nitrogen and has a strong, unpleasant odour. Ammonia, a nitrogen-hydrogen combination, can be produced either naturally or synthetically. It is used as a precursor in the production of a wide variety of nitrogen-based compounds with significant economic value.
Ammonia is an odorless, colorless gas with a distinctive taste and smell. It has several applications in the manufacturing sector and is considered a vital chemical.
Ammonia is a natural byproduct of the decomposition of organic things like decaying plants and animals. This gas is also discharged into the air when fossil fuels are burned.
Ammonia molecule
Some characteristic physical properties of ammonia are:
Ammonia is an odourless gas that has a strong, penetrating smell.
It has a freezing point of 77.7 °C (107.8 °F) and a boiling point of 33.35 °C (28.03 °F).
The substance has a high heat of vaporization (23.3 kJ/mol) .
A lone pair is located on the ammonia molecule's nitrogen atom, giving the molecule a trigonal pyramidal form.
A strong intermolecular hydrogen bonding makes this polar compound strongly linked.
A superior solvent for organic compounds than water, ammonia has a dielectric constant of only 22.
Ammonia's pKa isn't quite as high as some other ionising solvents, yet it's significant enough to be useful. Like water, ammonia undergoes a weak self-ionization process, as follows:
2NH3 ⇌ NH4+ + NH2−
Some common uses are:
Ammonia is mostly used as a fertilizer. Liquified gas is typically poured straight onto the ground from storage tanks in the United States. Salts of ammonia, like ammonium nitrate (NH4NO3), ammonium sulfate (NH4S2O4), and different ammonium phosphates, are also acceptable forms of ammonia. The nitrogen compound urea (NH2)2CO is widely utilized as a fertilizer ingredient worldwide.
A further application for ammonia is in producing industrial pyrotechnics (nitroglycerine, TNT, nitrocellulose, etc.).
Ammonia is crucial in producing man-made fibers like nylon and rayon in the textile industry. It's also used to bleach and scrub natural fibers like wool, linen, and silken. Certain synthetic resins can be made using ammonia as a catalyst. Most importantly, it inhibits the coagulation of crude latex during transit from the plantation to the factory in the rubber sector and neutralizes corrosive by-products of petroleum refining.
Two processes utilize ammonia: the Ostwald process, which transforms ammonia into nitric acid, and the Solvay process, commonly used to produce soda ash.
Nitriding alloy sheets use ammonia to stiffen their surfaces and are only one of several metallurgical applications for the chemical. As ammonia can be broken down into hydrogen gas, it makes for a great portable supply of atomic hydrogen that may be used for welding.
1 gm of ammonia absorbs approximately 320 calories of heat. Thus it is widely used as a refrigerant.
Last but not least, it's sometimes found in household cleaners.
Joseph Priestley, an English physicist, created the first pure ammonia in 1774, and in 1785, Claude-Louis Berthollet, a French chemist, identified its precise composition. The United States routinely ranks ammonia production among its top five chemical outputs. Ammonia is mostly manufactured by the Haber-Bosch process because of the direct interaction of hydrogen and nitrogen.
N2 + 3H2 → 2NH3
High pressure (200-11,000 atm) and heat (500-600 °C) are needed for this reaction to proceed.
In reality, low temperatures favor ammonia creation because of the balance between the elements and ammonia, but high temperatures are necessary to generate ammonia at an acceptable pace.
It is possible to utilize a wide variety of catalysts. The catalyst often used is iron oxide. Nevertheless, ruthenium on carbon and magnesium oxide on aluminum oxide, the latter being the one that was activated by alkali metal oxides, have also been used as catalysts.
Ammonia is an odorless, colorless gas having a very distinctive taste and smell. It has a wide range of applications in industry, making it among the most crucial compounds. Ammonia, a nitrogen-hydrogen combination, can be produced either naturally or synthetically. Ammonia has several applications in industry. In several applications, ammonia proves to be an indispensable ingredient. Due to its ability to improve plant nutrient uptake, it is a vital component of fertilizers. It's also a key ingredient in making things like plastics, explosives, and medicine. Due to its non-toxicity and low boiling point, ammonia is also employed as a refrigerant.
Intense freeze burns to the skin, eyes, throat, sinuses, and lungs can develop from contact with ammonia. Dress in layers, including long sleeve shirt and slacks, and wear protective gear such as NH3-rated safety glasses and gloves with cuffs (no shorts). Never put on contact lenses. Every nurse floater has to have, at minimum, a 5-gallon water container in case of an emergency.
The spaces used for storage and transport must have enough ventilation and be free of oxidizers, combustibles, high temperatures, and sparks. Bulk storage tanks that don't require refrigeration can store ammonia, along with shipping cylinders. It's important to take precautions against container overfilling.
Smoking and open flames are forbidden in areas where ammonia is used, handled, or stored because they provide a fire or explosion risk even though ammonia does not readily ignite (its flammability danger is minimal).
