We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
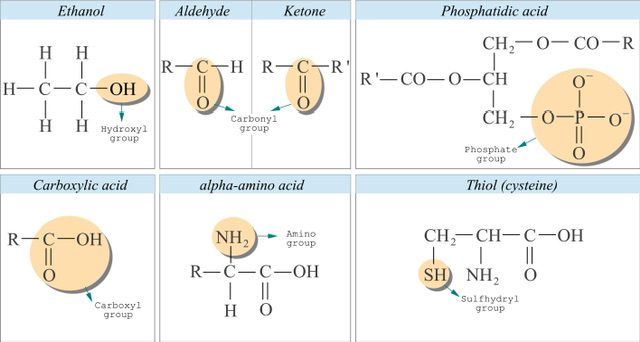
Aldehydes, Ketones, and Carboxylic acids are all organic compounds with different functional groups. The structure RCHO represents an aldehyde while the structure RCOR represents ketones and RCOOH or RCO2H represents carboxylic acid. Aldehydes possess a central carbon atom and which is then connected with a Hydrogen atom through a single bond and through a double bond to Oxygen atom.. Ketones contain a central carbon atom and are connected with Oxygen by a double bond and then through a single bond to the carbon atom. Carboxylic acids contain a central carbon atom connected to an OH group with a single bond, Oxygen with a double bond, and connected to another carbon atom through a single bond.
Carboxylic acids are carbonyl compounds. They are organic acids. The most common example of a carboxylic acid is acetic acid or vinegar. The general representation of carboxylic acid is RCOOH. These carboxylic acids are found in many animals and plants. Fruit such as oranges and lemon contains citric acid. Through the process of fermentation, lactic acid is produced and is found in applications in the food industry. Methanoic acid, ethanoic acid, and propanoic acid are the first three carboxylic acids.
Carboxylic acid contains a central atom bonded through a single bond to OH and an oxygen atom through a double bond. And also to another carbon atom.
Some examples of carboxylic acid are acetic acid, propanoic acid, benzoic acid, Valeric acid, palmitic acid, stearic acid, etc. Carboxylic acid is found naturally in some plants and animals. In plants, citric acid is found. In animals and plants, fatty acids are present. Some of the fatty acids are oleic acid, linoleic acid, etc. And amino acids are present in animals. Butanoic acid is produced in the sweat glands. And amino acids are compounds that contain amino groups. Some of the amino acids found are histidine, isoleucine, tryptophan leucine, lysine, phenylalanine, threonine, methionine, and valine.
Carboxylic acids are colorless compounds. They have a sour taste. The higher carboxylic acids are solid at room temperature while lower carboxylic acids are liquid at room temperature. The boiling and melting point are increased with an increasing number of carbon atoms present in the compound. They are also less soluble in water. And the solubility is found to decrease by increasing the number of carbon groups present in the alkane part. But are soluble in toluene, ethanol, and diethyl ether. The pure form of water-free acetic acids is called glacial acetic acid.
The naming is done in a way that the alkane group is named with the IUPAC nomenclature and then at the end the suffix 'oic acid' is used. For example, CH3-COOH is named ethanoic acid and C6H5-COOH is named benzoic acid since a benzene group is attached. For amino acids, the substituent for amino groups is also given. For example, NH2-CH2-CH2-COOH ( 3- Amino propanoic acid). The number for the amino group is done by counting it from the carboxyl carbon. In this way, naming is done.
They possess a high boiling point and a high molar mass due to the presence of strong hydrogen bonding. And this is because of the presence of hydroxyl groups present in the compound. The hydrogen bonding present on them is,
They are weak bronsted Lowry acids. Since they can lose one proton present in the compound. And the acidity varies with different alkyl groups. For oxalic acid, the pKa value is 1.27 and for benzoic acid, the pKa value is 4.2. Carboxylic acid loses one proton present in the compound and forms a carboxylate anion that is RCOO- .
Carboxylic acids are used in the food industry for example vinegar is used as a preservative.
The carboxylic acid ethanol acid is used in the medicine aspirin.
It is used in the manufacturing of perfume and artificial essence.
Salicylic acid is used in acne creams.
Butanoic acid is used in the manufacture of toothpaste, mouthwash, and lipstick.
Methanoic acid or formic acid is used for coagulating the latex of rubber trees.
Carboxylic acids are used in the soap and detergent industry.
It is used in the production of polymers and adhesives.
Aldehydes, ketones, and Carboxylic acid are carbonyl compounds with CO as their common functional group. The nomenclature of aldehydes, carboxylic acid, and ketones are almost the same. The Ketones suffix is 'one' while for Carboxylic acid is 'oic acid' and for an aldehyde is 'al'. Carboxylic acid has a colorless appearance and a sour odor. And is a weakly acidic compound. The use of carboxylic acid is also very wide; it has found applications in the food, pharmaceutical, cosmeceutical, and polymer industry. Among the carboxylic acids, acetic acid or vinegar is commonly known and is used as a preservative in the food industry. The use of carboxylic acid is very wide.
1. Which is more acidic out of Carboxylic acid and phenol?
Ans: Carboxylic acids are stronger acid even if it contains an OH group as the functional group. This is because, in the carboxylate ion, the negative charge is always on an electronegative atom (oxygen). While in the phenolate ion, a negative charge is on the C atom as well as the O atom.
2. Which is the stronger acid CH3COOH or CCl3COOH?
Ans: The three Cl atoms in the CCl3COOH molecule increase the electron-withdrawing effect and stabilizes the carboxylate ion. Thus it is more acidic in nature than CH3COOH.
3. Why is COOH electron-withdrawing?
Ans: This is because of the presence of the hydroxyl group or OH group since it contains an Oxygen atom it is a highly electronegative compound so it will act as an electron-withdrawing group. Hence the COOH group in carboxylic acid is electron-withdrawing.
