We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
Introduction:
Greek words chroma, which means "color," and graphein, which means "to write," are the roots of the word "chromatography." For separating a mixture, it is applied to a stationary phase (liquid or solid) in this technique, and a pure solvent like water or a different gas is enabled to travel gradually through the stationary phase. It transports the individual components according to the respective solubility in that solvent. This pure solvent is called the mobile phase. In the food industry, chromatography is utilized during the manufacture of beverages.
In this separation method, the analyte is mixed with either liquid or gaseous mobile phase that is once again pumped through a stationary phase. Generally, the two phases are hydrophilic and lipophilic.
Two phases are then made to interact with the constituents of the analyte. According to their polarity, they come in contact with the stationary phase for a shorter or maybe longer amount of time, which delays them accordingly.
All the components that make up the sample are separated. Each constituent of the specimen is extracted from the stationary phase at a specific time known as the retention time.
By tracking and graphing the output signals of the constituents as they pass through the detector, a chromatogram is produced.
Separation by chromatography
There are primarily four kinds of chromatography.
Adsorption Chromatography:
Different compounds are adsorbed on the adsorbent at a greater or lower extent in the adsorption process, based on the component's absorptivity. The components having stronger absorptivity are carried to a relatively shorter distance than those with lower absorptivity. The mobile phase remains over the stationary phase and this mobile phase contains the components.
Column Chromatography:
Column chromatography is a method that separates the elements of a mixture by utilizing a column of appropriate adsorbent filled in a glass tube. As soon as the mixture is poured on top of the column, an appropriate eluent is developed to stream slowly through it. Depending on the number of individual constituents that have been adsorbed to the wall of the adsorbent column, the components are separated. The most absorbent element is kept at the top, and the rest of the elements flow down to varying heights in accordance.
Column chromatography
Thin Layer Chromatography:
The thin-layer chromatography (TLC) procedure uses a glass plate covered with an extremely thin layer of an adsorbent, such as alumina or silica gel. It is used To separate the non-volatile mixture into its constituent parts. The sheet used in this process is known as the chrome plate. To begin with the process of separation, a small portion of the mixture's solution is placed 2 cm over one side of the plate. The plate is then placed within a container that is tightly shut and loaded with an eluent. As the fluid rises up the plate, it lifts various constituents of the mixture to different heights.
Partition Chromatography:
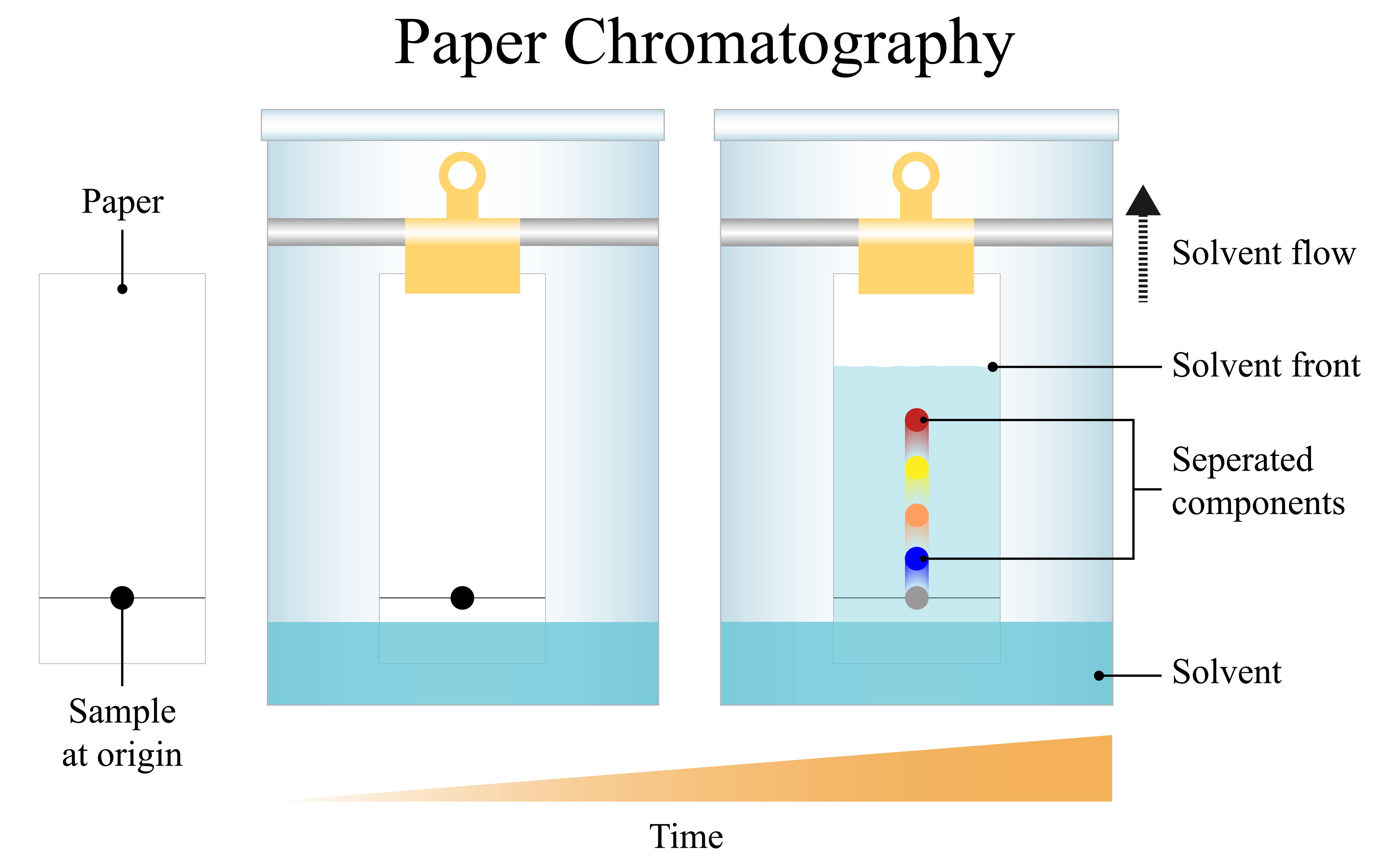
A constant differential separation of mixture components in a stationary phase, as well as a mobile phase, occurs in this process. A constant differential separation of mixture components in a stationary phase, as well as a mobile process, occurs in this process. Partition chromatography is nicely illustrated by paper chromatography. Chromatography paper serves as the procedure's stationary phase and is itself suspended in a mixture of solvents, which serves as the procedure's mobile phase.
Depending on how strongly the constituents adhere to the paper, the mixture is placed in a location at the bottom of the chromatography paper, and when the solvent raises through the paper, the components are transferred to varying degrees. The components are consequently split at varying distances.
The ratio of the spot's elevation from the baseline to the height of the solvent front above the baseline is called the retention factor for a given substance.
Polarity and density of solvents: More polar solvents decrease the retention factor value. If the solvent is not viscous at all, it will increase the efficiency of separation.
Temperature: With the increase in the column temperature, increase the rate of elution. But it can’t improve the process.
Size: Small size of particles in the stationary phase gives more surface area which improves the separation process.
Dimensions of the column: The separation process becomes efficient if the ratio of height to the width of the column increases.
By assisting in the assessment of the period at which food expires, the chromatography technique plays a crucial role in the food processing industry. Moreover, this method can be used to indicate the existence of chemical additives in the food.
Chromatography is essential in the chemical sector for determining the purity of water samples.
In the chemical synthesis industry, various techniques of chromatography are often used to analyze air samples for purity.
Numerous chromatographic techniques are frequently used in the molecular biology field of proteomics and metabolomics.
In applications involving protein isolation, one kind of chromatography is classified as HPLC (High-Performance Liquid Chromatography).
Chromatography makes it possible to separate, assess, and purify things precisely.
Very small samples can be separated using this technique.
It works well with a varied range of samples which includes food particles, plastics, water, and air samples, tissue extracts, and medications.
A mixture that is separated by chromatography, allows for the recovery of every component. Using this technique, highly complex mixtures can be separated.
This separation process of substances is a laborious procedure.
Because more solvents are needed, it is more expensive.
Its separation capacity is weak.
Chromatography is an analytical method used for the separation of components in a mixture. At first, the mixture becomes soluble in a material known as the mobile phase, which then transports it through a substance known as the stationary phase. Based on the value of retention factors of different constituents, all the components of a mixture are separated. Chromatography's goal is to distinguish between the various components of a mixture. This method has a great application in the purification of water, in the chemical and food industries, and many others. Despite some disadvantages, it is still employed in almost every laboratory and industry for separation purposes.
What are the forces used in chromatography?
Ans: The London dispersion, dipole-dipole, and ion-dipole forces are the four forces used during chromatography.
What kind of solvents does chromatography often use?
Ans: In chromatography, low-viscosity solvents are typically used. This is caused by the fact that a solvent's speed of flow changes inversely with respect to its viscosity. The solvents like DCM, petroleum ether, and ethyl acetate can be used based on the polarity of the components in a mixture.
What kinds of substances can be partitioned using chromatography?
Ans: For the separation of extensive combinations of proteins, amino acids, organic molecules, drugs, carbohydrates, and a large variety of simple chemical compounds, paper chromatography has evolved into a common procedure. Inorganic ions can also be easily separated.
