We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
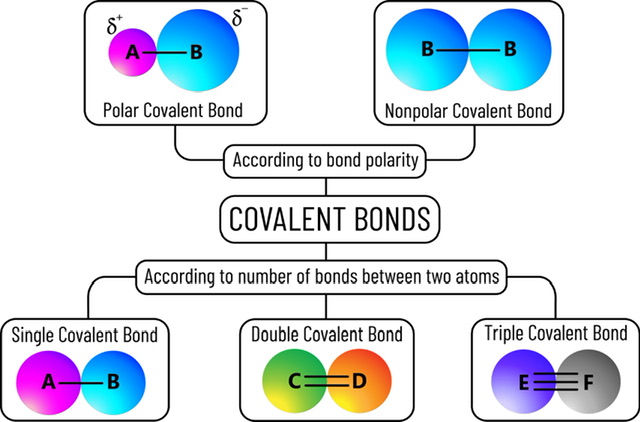
When atoms share pairs of electrons, a phenomenon known as covalent bonding takes place. By establishing a complete electron shell through covalent bonding, atoms can become more stable. Atoms become more stable when their outermost (valence) electrons are shared, allowing them to fill their shell. To achieve stability, nonmetals often create covalent bonds with one another; depending on their valence electron count, these bonds can range from one to three. When atoms create covalent connections, it is often not the case that they share electrons evenly.
Each atom in a stable molecule must have 8 valence electrons, which can be achieved by electron transfer, loss, or gain, as per the Octet Rule. Covalent bonds often include atoms sharing electrons to conform to the Octet Rule. The s- and p-orbitals (electronic configurations) in a noble gas are filled with 8 electrons, hence this state of affairs necessitates the addition of 2 more electrons. As noble gases are neutral, every atom strives to achieve the same stability as these substances. It's vital to keep in mind the number “8”, however, there are several exceptions to the Octet rule.
When two atoms share an electron pair, the resulting link is called a single bond. A single line connecting the two atoms represents this. This type of bond is considered to be the most stable since it is less reactive than the double and triple bonds it is weaker than and has a lower density.
For example, in HC there is just one bond in hydrogen chloride, as shown in the Lewis dot structure below. There is one hydrogen atom and one chlorine atom in hydrogen chloride, as can be seen in the chemical formula below. Hydrogen only contains one valence electron, but chlorine has seven. For a single bond to form, the Octet Rule requires that each atom give up one of its electrons to the others.
HCl molecule
Double Bonds
Two atoms form a double bond if they share four electrons, 2 electrons by each atom. Symbolized by two parallel horizontal lines connecting the two constituting atoms of the molecule. Double bonds are always stronger than single bonds which leads to their short length owing to more force of attraction between the bonded atoms. They are, however, more reactive than single bonds or addition reactions. An illustration of a double bond in carbon dioxide's Lewis dot structure is shown below. Carbon dioxide, as can be seen in the image below, consists of one Carbon atom and two Oxygen atoms. There are six valence electrons in an oxygen atom but only four in a carbon atom. Carbon requires an additional 4 valence electrons to conform to the Octet Rule. Since Oxygen has three unshared electron pairs, it may donate one pair to Carbon, filling the latter's valence shell.
CO2 molecule
When two atoms in a molecule share electrons in three different pairings, the link is called a triple bond. It's the weakest of the three standard kinds of covalent bonding. It is very reactive towards nucleophiles. A common example is acetylene.
When atoms exchange electrons but not in equal amounts, a polar covalent bond forms. This occurs when the electronegativity of one of the atoms in a bond is greater than that of the other atom. More electrons will be attracted to the atom with greater electronegativity. Because of this, the electrons that are shared will be clustered around the atom with greater electronegativity, leading to an uneven distribution of electrons. As in a game of Tug-of-War, the stronger player typically prevails.
Examples: HCl, NH3, H2O, etc.
When atoms give and take electrons in a balanced fashion, a nonpolar covalent bond forms. This typically happens amongst atoms that share a comparable or same electron affinity. The greater the similarity between their electron affinities, the stronger their attraction. Gas molecules, sometimes called diatomic elements, undergo this transformation. The notion of nonpolar covalent bonding is very similar to that of polar covalent bonds in that the atom with a greater electronegativity would steal an electron from the atom with a lower electronegativity. As all the atoms in our diatomic molecules are the same sort of element, their electronegativity will be identical, and the charge on the molecule will be zero if we apply this statement to it (i.e., a nonpolar covalent bond).
Example: H2, Cl2, etc.
1. For what kind of atoms do covalent bonds form?
Sharing of electron pairs between atoms and other covalent connections characterizes the chemical bonding known as covalent bonding, which occurs between nonmetallic atoms.
2. What causes covalent compounds to melt so quickly?
In covalent compounds, intramolecular forces are too weak to keep the molecules together. Hence, the energy necessary to separate two or more molecules is negligible. This explains why their boiling and melting temperatures are so low.
3. What are covalent bonds such as bad electrical conductors?
As electrons are exchanged during bond formation, there is no number of electrons or ions present for electricity conduction. This is why covalent bonds are such bad electrical conductors.
