We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
Both esters and ethers are considered to be functional groups in the category of organic chemical compounds. Classifying chemicals allows us to examine their features in the larger context of the group to which they belong. Both esters and ethers belong to functional groups of chemicals that find widespread use in industry and human life.
Esters and ethers are structurally different compounds.
To fully form its characteristic structure, an ester group needs two carbon atoms and two oxygen atoms, whereas an ether group just needs one of each. It just takes 1 oxygen atom and 2 carbon atoms to form an ether group.
It is an organic compound with two alkyl or aryl groups bonded to the oxygen atom (O). Alkyl ethers are the simplest type of ether, consisting of just two very small alkyl groups connected to an O atom. The alkyl groups must be mentioned in alphabetical order in their nomenclature, and the word "ether" must be included at the conclusion.
An n-butyl methyl ether, for instance, is an ether that also has a methyl group linked to an O-atom. It's true that ethers may dissolve a broad variety of materials, both polar and nonpolar.
Most importantly, this is because there is no network of hydrogen (H) bonds that must be broken in order for the solute to be dissolved.
It has an O-atom that is connected to 2 alkyl or aryl groups. The expected shape of ethers should be tetrahedral. The oxygen (O) in ether has a sp3 hybridization, as well as the 2 lone pairs, are in 2 hybridized orbitals, with 2 participating in bonding with R groups. R-O-R'.The bond angle is around 104.5°, which is comparable to water (H2O).
Ethers are widely used as commercial solvents
They are also used as a cooling agent
Aestheticians used ether in surgery.
It is also used along with petrol as a motor fuel
It is very common to use it as a solvent in laboratories
It is used for extracting organic compounds.
In Grignard reactions as a solvent to stabilise the reagent.
It is the act or process of producing ether; especially, the transformation of a large volume of alcohol into the ether with the use of a tiny portion of sulfuric (H2SO4), or ethyl sulfuric, acid. This is often done with aliphatic and aromatic alcohols (phenols). In the situation of simple aliphatic alcohol, acid catalysis produces the ether. The Williamson synthesis is utilized in more difficult instances for several phenols:
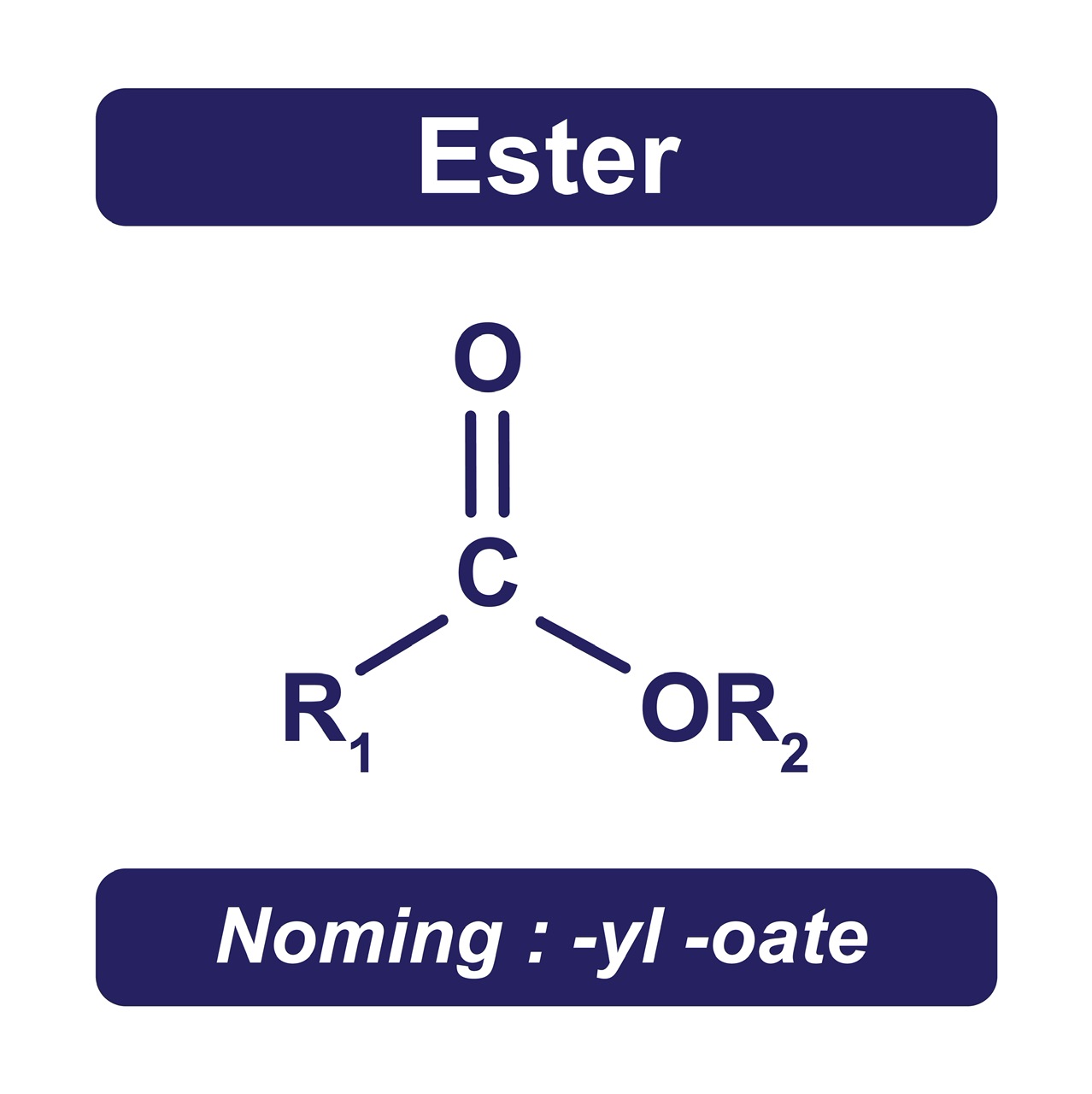
It is an organic product formed when an oxoacid reacts with a hydroxyl component (such as alcohol or phenol). It is similar to a carboxylic acid in that the hydrogen (H) atom of the -COOH group has been replaced with an alkyl or aryl group. Since esters cannot create hydrogen (H) bonds with one another. They can, however, generate H-bonds between the O-atoms in their bodies as well as the H-atoms in water (H2O) molecules. As a result, esters are only marginally soluble in water (H2O).
Additionally, unlike the comparable carboxylic acid, an ester has a fruity odor. Indeed, they are responsible for the odor of many fruits; for instance, ethyl ethanoate gives pineapple its odor. As a result of this phenomenon, esters are now used in the food sector.
However, the esters utilized in a product to provide the desired fruity fragrance are not the same substance found in nature. Nonetheless, it can have the same flavor but also odor. Furthermore, while the chemical is not the same as in the original fruit, eating these food products is not harmful since the ester structure is quite similar to that of the natural substance.
It has one O-atom which is double bonded to a C-atom which is again connected to O-atom which is linked to C-atom. They are polar compounds, although their boiling temperatures are less than those of equal-weight carboxylic acids. They exhibit tetrahedral geometry and the central C-atom of ester has sp3 hybridization.
As a flavouring in the food industry.
It is also used as a perfume and fragrance.
It serves as a solvent for paints, varnishes and inks.
In making plastics and synthetic fibres like polyester.
Ester is also used in pharma as a part of drug formulations
Ester are also used in manufacturing biodiesels.
Production of soaps and detergents also uses esters
It is also used in the cosmetic industry.
Ester formation is an equilibrium reaction between an alcohol and a carboxylic acid. Similarly, acyl chlorides (acid chlorides) and alcohols, or acid anhydrides and alcohols, can be used to produce esters. In the presence of alcohol, carboxylic acid reacts to generate an ester. There are three distinct mechanisms through which esterification can take place.
by adding ethanol to carboxylic acid.
by combining acid chloride with alcohol
by reacting alcohol with acid anhydride
The primary distinction between ester vs ether is that ester is an organic product created by the interaction of carboxylic acid with an alcohol, whereas ether is an organic complex composed of carbon (C), and oxygen (O), and carbon (C) atoms. An ester is an organic molecule formed by the reaction of an oxoacid with a hydroxyl component. An ether, on the other hand, is an organic molecule that contains an O-atom in addition to 2 alkyl or aryl groups. The primary distinction between ester as well as ether is that ester has a functional group of -COO, whereas ether has a functional group of -O.
To build a strong foundation in science, it is important to know concepts like Ester and Ether. At 88tuition, we help students with these basic concepts. In our PSLE Science Tuition, we provide clear video lessons and concept explanations. For PSLE exams, we make sure that a child develops a deep understanding of science. Our structured approach provides benefits to the children to excel in their exams.
Ethers are highly sensitive to light because they produce explosive peroxides when combined with oxygen (O) in the presence of light. Because ultraviolet light (UV) is harmful to ethers, they must be stored in amber containers, which are brown in color.
Esters, carboxylic acids, amides, and other functional groups containing carbonyl groups are reduced by LiAlH4. It does not affect non-polar bonding. Ethers have nonpolar bonding and no carbonyl group. As a result, it cannot be decreased by LiAlH4.
Transesterification involves the replacement of the ester's organic functional group R′′ with the alcohol's organic functional group R'. A catalyst, often an acid or a base, is added to speed up these reactions.
4. Is Ether the same as ester?
No. Ester and Ether are different compounds. Ester has a sweet, fruity smell and forms from acid and alcohol reactions. Ethers form when molecules do not create hydrogen bonds and have no odour.
5. What is ether called now?
Ethers are still known as ethers in chemistry. It refers to a class of organic compounds characterised by an oxygen atom with two alkyl groups.
