We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
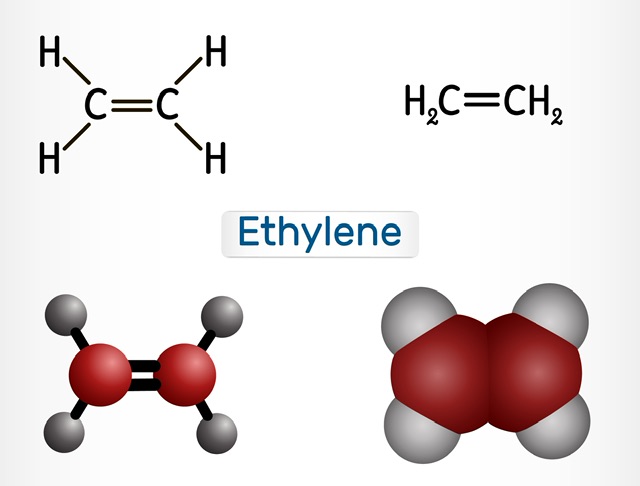
Ethylene, often known as ethene, is an odorless gas that can catch fire. It is one of the simplest unsaturated hydrocarbons, containing only two carbon atoms and four hydrogen atoms. A lot of ethylene is produced for many industrial uses, including the production of plastics, synthetic rubber, and chemicals. Moreover, it affects a variety of biological processes, including fruit ripening and plant growth. Due to its double bond, ethylene is extremely reactive and can undergo addition polymerization to produce polyethylene, the most commonly used plastic worldwide. Let us study its polymerization in detail.
Structure Of Ethylene
Polymerization & Types Of Polymerization
Little molecules called monomers combine to form a bigger molecule called a polymer during the chemical reaction known as polymerization. The double or triple bonds between the atoms in the monomers must be broken to form long chains or networks.
Different types of polymerization include:
Addition Polymerization: In addition to polymerization, unsaturated monomers like ethylene are added to form a polymer chain. A catalyst, such as a complex of a transition metal, triggers it. The carbon-carbon double bond of the monomer is broken during addition polymerization, which is followed by the formation of a new single bond between the two carbons on neighboring monomers, releasing a small molecule, such as hydrogen gas.
Condensation Polymerization: Condensation polymerization includes the elimination of a tiny molecule during the polymerization process, such as water or alcohol. Materials like nylon and polyester are examples of condensation polymers. Condensation polymerization is a process that creates polymer chains out of two distinct monomers that have different functional groups, such as carboxylic acid and alcohol groups, as well as tiny molecules like water.
Ethylene can be polymerized using a variety of methods, including high-pressure free-radical polymerization, Ziegler-Natta polymerization, and metallocene polymerization.
High-pressure free-radical polymerization is the most typical method for creating polyethylene, a linear thermoplastic polymer. High pressure and a radical initiator are employed to start the reaction, ensuring that the monomers are close enough to one another for the reaction to happen. The process is quite simple and inexpensive, and it has the potential to produce polyethylene with a wide range of molecular weights and properties.
where n is the number of repeating units in the polymer chain.
Another common method for creating polyethylene is ziegler-natta polymerization. In this method, the reaction is initiated by an organo-aluminium molecule and a transition metal complex known as a Ziegler-Natta catalyst. The catalyst's capacity to control the molecular weight and structure of the polyethylene could result in a variety of polyethylene types with distinct structures resulting in several kinds of polyethylene with distinctive properties, including linear low-density polyethylene (LLDPE), high-density polyethylene (HDPE).
Monomer to Polymer through Polymerization
Mechanism
The ethylene polymerization mechanism consists of the initiation, propagation, and termination of polymer chains.
Initiation: An activator, such as a Ziegler-Natta catalyst, kickstarts the polymerization process. The catalyst is frequently a compound of a transition metal, such as titanium tetrachloride, that can coordinate with the ethylene monomers.
Propagation: The extending polymer chain may engage in ethylene monomer reactions once the catalyst initiates the polymerization reaction. The catalyst takes an ethylene monomer from the growing chain and coordinates it with a brand-new one. As a result of this continuing process, a polymer chain develops.
Termination: The polymerization process can be stopped by a variety of methods, including disproportionation, beta-hydrogen elimination, and chain transfer.
A typical industrial process known as ethylene polymerization produces a wide variety of polyethylene polymers with different properties and purposes. Some of the primary applications for ethylene polymerization include the following:
Packaging: One of the primary applications for ethylene polymerization is the production of polyethylene films and bags, which are widely used in packaging. High-density polyethylene (HDPE), which is robust, durable, and resistant to tears and punctures, is particularly well-suited for packaging applications.
Pipes & Fittings: Polyethylene pipes and fittings, which are widely utilised in the construction industry for purposes including gas distribution, drainage, and water delivery, are also produced through the ethylene polymerization process.
Wire and cable insulation: For applications involving wire and cable, polyethylene insulation is also produced through the ethylene polymerization process. Low-density polyethylene (LDPE) and linear low-density polyethylene (LLDPE) are widely used in these applications due to their excellent electrical properties and low dielectric constant.
Eventually, ethylene polymerization, a fundamental procedure in the study of chemistry, is the joining of ethylene monomers to produce polyethylene polymers. A range of polyethylene goods, including pipes, wire insulation, auto components, medical equipment, and packaging materials with various physical and chemical properties, must go through this process in order to be produced. Ethylene polymerization is a key component of modern industry, and ongoing research in this area aims to improve the efficiency and sustainability of the process while extending the variety of possible uses.
1. What are the advantages of using single-site catalysts for ethylene polymerization? More control over the molecular weight and structure of the resultant polymer is provided by single-site catalysts, which can enhance its performance and characteristics.
Co-monomers like butene or hexene can alter the properties of polyethylene by altering the molecular weight and branching of the polymer. Co-monomers can also improve the thermal and mechanical properties of the resulting polymer.
3. How is the quality of polyethylene produced through ethylene polymerization evaluated?
Polyethylene quality is determined by factors such as molecular weight, density, melt flow rate, heat stability, and mechanical properties. These qualities can be evaluated using a variety of analytical methods, including tensile testing, differential scanning calorimetry, and gel permeation chromatography.
