We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
Certain amino acids and monosaccharides have simple, straight-chain structures that are most accurately represented by Fischer projections. These are symbolic representations of the shapes that may be used to effectively communicate stereochemical information by depicting three-dimensional molecules on a two-dimensional plane. Such structures have the benefit of being able to represent several stereocenters and make the identification of planes of symmetry straightforward.
Fischer projections are named after Emil Fischer, who developed them in 1891. Fischer projections are used to represent the three-dimensional structure of monosaccharides in a two-dimensional form. This makes it easier to compare and analyze the structures of different monosaccharides. Fischer projections are composed of vertical and horizontal lines. The vertical lines represent the carbon atoms, while the horizontal lines represent the bonds between the carbon atoms. The carbon atoms are numbered from 1 to 6, starting from the top and going clockwise. The bonds between the carbon atoms are represented by arrows pointing either up or down. The direction of the arrows indicates the direction of the bond. Fischer projections can also be used to represent the stereochemistry of compounds. Stereochemistry is the study of the three-dimensional arrangement of atoms in a molecule. In Fischer projections, the stereochemistry is represented by the direction of the arrows. If the arrows point up, the molecule is in the L-configuration, while if the arrows point down, the molecule is in the D-configuration.
Monosaccharides are the simplest form of carbohydrates and are the building blocks of more complex carbohydrates. They are composed of single molecules of carbon, hydrogen, and oxygen atoms and are classified as either aldoses or ketoses. Monosaccharides are the most abundant form of carbohydrates in nature and are essential for life.
Aldoses are monosaccharides that contain an aldehyde group, while ketoses contain a ketone group. Aldoses are further divided into three categories: trioses, tetroses, and pentoses. Trioses contain three carbon atoms, tetroses contain four carbon atoms, and pentoses contain five carbon atoms. The most common aldoses are glyceraldehyde, dihydroxyacetone, and erythrose.
Ketoses are monosaccharides that contain a ketone group. They are further divided into three categories: tetroses, pentoses, and hexoses. Tetroses contain four carbon atoms, pentoses contain five carbon atoms, and hexoses contain six carbon atoms. The most common ketoses are fructose, ribose, and xylose.
Monosaccharides can be further classified based on the number of chiral centers they contain. Chiral centers are carbon atoms that have four different groups attached to them.
Fischer projections can be used to represent both aldoses and ketoses. Aldoses are monosaccharides with an aldehyde group, while ketoses are monosaccharides with a ketone group.
The aldehyde group is represented by a horizontal line at the top of the Fischer projection, while the ketone group is represented by a horizontal line at the bottom.
Monosaccharides (such as glucose and fructose) benefit from Fischer projections due to the high number of stereocenters (or carbons with unique bonds) present in their structures. Except the direction of their stereocenters, the various monosaccharides are quite identical to one another. All of the chiral carbons, hydrogens, hydroxyls, and amino groups are shown as crossed lines.
Fischer projections are a useful tool for analyzing and comparing the structures of different monosaccharides. They can also be used to represent the stereochemistry of monosaccharides, making them an invaluable tool for biochemists and other scientists.
Some common monosaccharides are:
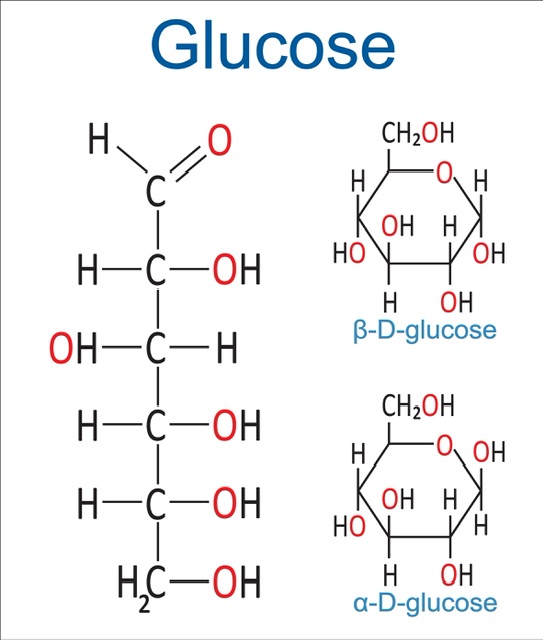
Glucose: Glucose, a monosaccharide sugar that comes in two forms (alpha and beta) and has the molecular formula C6H12O6, provides energy to cells in both plants and animals by breaking down oxygen into carbon dioxide and water.
Fischer Projection of Glucose
Galactose: Similar in sweetness to glucose and roughly 65% as sweet as sucrose, galactose is a monosaccharide sugar that is sometimes abbreviated as Gal. It is glucose's C-4 epimer and an aldohexose.
Galactose Fischer Projection
Mannitol: It is a polyhydroxy 6-C carbohydrate which is linear in structure. It is a low molecular weight compound. Its chemical formula is C6H14O6.
Fischer projection of Sorbitol and Mannitol
Sorbitol: It is an isomer of Mannitol and has the chemical formula C6H14O6. This is also a polyhydroxy six-carbon sugar which shows hydrogen bonding.
When trying to figure out how carbohydrates are put together, the Fischer projection of monosaccharides is a great resource. We can now see the three-dimensional structure of a monosaccharide in a two-dimensional representation. This facilitates the process of determining the stereochemistry of a molecule by locating its chiral centers. As a bonus, we may use it to swiftly ascertain the sugar's structure and locate the anomeric carbon. The Fischer projection may be used to rapidly determine the stereochemistry of a monosaccharide, making it a useful tool for studying carbohydrate structures.
Frequently Asked Questions
A Fischer projection of monosaccharides is a two-dimensional representation of a three-dimensional structure of a monosaccharide molecule, while a Haworth projection is a three-dimensional representation of a monosaccharide molecule.
A Fischer projection of monosaccharides is used to identify the chiral centers of the molecule and to determine the configuration of the molecule. This information can then be used to determine the configuration of the molecule.
Monosaccharides are single sugar molecules, while disaccharides are two sugar molecules bonded together. Monosaccharides are the building blocks of carbohydrates, while disaccharides are the end products of carbohydrate digestion.
