We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
A thermodynamic system is a body of matter that is confined by boundaries within a specific space. These systems can be categorized into closed, open, and isolated systems based on the type of boundaries that confine them. An isolated system as the name implies is completely isolated and does not exchange energy or matter across its boundaries. A closed system, on the other hand, allows for energy exchange but matter cannot move across its boundaries. Finally, an open system is one which permits the free exchange of energy and matters across its boundaries. The study of heat, work, temperature, and their relationships with energy, entropy, and other physical properties of matter falls under a branch of physics known as thermodynamics.
Latent heat is the energy absorbed or released by a substance when it undergoes a physical state change without changing its temperature. This concept was introduced by British chemist Joseph Black and is experimentally determined using a process known as calorimetry. Latent heat is the energy required to overcome the intermolecular forces between the atoms or molecules in a substance, which varies with the state of the material. For instance, gas molecules have greater molecular vibrations, while water molecules exhibit increased mobility when heat is applied.
Latent heat itself is categorized into the following types: latent heat of vaporization, latent heat of fusion, and latent heat of sublimation.
Latent heat of fusion
The energy required or released by a substance during the transition from a solid to a liquid state is known as the latent heat of fusion. When a solid material is heated at constant pressure, it melts and transforms into a liquid state. The amount of heat absorbed or released during this process is called the latent heat of fusion, which is determined by the change in enthalpy of the substance. For instance, ice absorbs heat and transforms into the water in liquid form.
Latent heat of vaporization
Latent heat of vaporization is the amount of heat energy required to transform a liquid into a gas or vapor at a constant temperature. For example, when water is boiled, it absorbs heat energy to transform into steam, which is an example of latent heat of vaporization.
Latent heat of fusion
Latent heat of sublimation
Latent heat of sublimation refers to the amount of energy that is absorbed or released by matter as it transitions directly from a solid state to a gaseous state, without turning into a liquid in the process. For example, the latent heat of sublimation for ice at 0℃ is 2,838 kJ/ kg.
Variation of heat with temperature
Specific latent heat of any type refers to that latent heat calculated for one kilogram of the material.
Mathematically, it is expressed as,
Here, Q denotes the amount of energy absorbed or released by the M mass of the material.
When energy is provided to water in the form of heat, its temperature starts to increase and it attains its boiling point of 100℃. Once there, the temperature stays constant, and providing further energy causes it to be converted to steam. Mathematically, we have,
By knowing the power of the source, we can calculate the specific heat of the water.
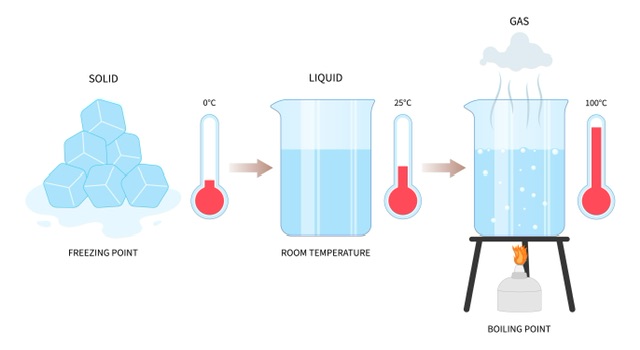
When a material is heated by an external source, it undergoes a change in its state during which its temperature remains constant. This is because the transition from one state to another requires additional energy. For example, when water is heated, its temperature rises until it reaches 100℃, and then remains constant until all the water has boiled off into a vapor. Similarly, when water is cooled, its temperature drops to 0°C and then remains constant until all the water freezes. This phenomenon is common across all forms and types of matter, as energy is always required to change the material's state, which keeps the temperature constant during the process.
Variation in states of water with temperature
This article discussed the concept of latent heat, which is the energy required to change the state of a particular substance at a constant temperature. Further, it discussed specific latent heat with special emphasis on the specific latent heat of the water.
1. What is the first law of thermodynamics?
The first law of thermodynamics relates to heat, internal energy, and work done. It is related to the law of conservation of energy and is mathematically stated as:
2. What is the law of conservation of energy?
The law of conservation of energy states that energy can never appear or disappear out of nowhere. It is never created or destroyed in a process. Rather, it gets converted across its different forms. For instance, heat energy given to an object does not vanish. Instead, it radiates that heat along with some radiation.
3. Thermodynamically, what makes steam so useful?
Steam has a very high specific latent heat capacity, which gives us the following advantages:
Boilers utilize steam. Since it has high specific latent heat, it can emit a large amount of heat energy and heat the room.
Steam is used for the conversion of chemical energy into coal since 1g of steam can yield as much as 2260 J of energy.
4. Give examples where latent heat is involved?
Due to latent heat, hurricanes get stronger. The air passing over warm water gets warmer and rises, holding water vapor and forming clouds. These clouds can then lead to a storm
Water freezes faster than air, a concept used in refrigerators.
5. What is the difference between specific and latent heat?
Specific heat is the amount of energy required to raise the temperature of one unit mass of a substance by one unit. On the other hand, latent heat is related to the change in the state of matter.
