We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
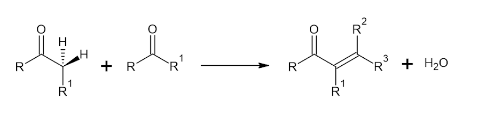
The aldol condensation originally comes from aldol (3-hydroxybutanal), which Wurtz established when he first produced beta-hydroxy aldehyde using acetaldehyde in 1872. Aldol condensation reactions usually involve self-condensations and often mixed condensations of aldehydes as well as ketones that result in β-hydroxy aldehydes and ketones, but also reactions that result in alpha, beta-unsaturated aldehydes and ketones composed by dehydration of approximate β-aldols as well as β-ketals. Various additional condensations featuring the reaction of an aldehyde and ketone also have been given the title aldol condensation. Crossed aldol condensations involving various carbonyl reactants are known as mixed reactions, even though they are successful under particular circumstances.
An enolate ion combines with such a carbonyl component throughout the presence of either an acid or base catalysts and generates a beta-hydroxy aldehyde as well as beta-hydroxy ketone, which is then dehydrated to yield a conjugated enone. Aldol condensations seem to be significant in organic reactions as it allows for the formation of C-C bonds. But in the most common form, it entails the nucleophilic addition of such a ketone enolate to that of an aldehyde and produces a β-hydroxy ketone, often known as an "aldol", a molecular unit present in several natural compounds as well as medications.
Applications
The process is employed in the gluconeogenesis as well as photosynthesis processes to produce a C-C bond.
It has several uses and importance in the study of metabolic biochemistry, but seems to have practical uses in the way of glycolysis, in which it serves to terminate the carbon-carbon end, and contradicts its function in the implementation of gluconeogenesis.
It is often used to make alcohol, isophorone, as well as diacetone.
Furthermore, it is also used as an intermediary in the perfume manufacturing method.
Used in the production of pharmaceuticals.
It is also utilized in the manufacture of insecticides.
It is utilized to make unsaturated ketones as well as chalcones.
This reaction has 2 components: an aldol reaction and dehydration—an eliminating process (including loss of water or an alcohol molecule). If there is an active carboxyl group available, decarboxylation will occur. The aldol addition reagent could be desiccated in 2 ways: the enolate process using a strong base such as NaH, KOH, as well as C4H9KO (potassium t-butoxide), and an acid-catalysed enol method. Due to the structure of the intended product, aldol condensation can be performed through one of 2 cases: kinetic control and thermodynamic control.
Crossed Aldol Condensation
Cross aldol condensation occurs as condensation among two distinct carbonyl compounds. Even when both include alpha-hydrogen atoms, this will produce a combination of 4 compounds. This is because both may create carbanions and function as carbanion acceptors. When one of the aldehydes lacks α hydrogen, it would only perform like a carbanion acceptor. Just 2 products have been developed in this situation. An aromatic aldehyde with no alpha orientation is a typical reagent for the cross aldol. Furthermore, the initial condensation reaction dehydrates quickly, resulting in the synthesis of alpha and beta-unsaturated ketones as well as prohibiting the retro aldol reaction from occurring.
Example of Cross Aldol Condensation
Benzaldehyde could be especially combined with several other alpha hydrogen aldehydes, as well as ketones.
Types of Condensation
Aldol condensation is distinct from other carbonyl compound reactions:
An aldehyde, as well as an aliphatic nitro molecule, are used in the Henry reaction.
Claisen Condensation contains 2 ester constituents.
Removal of water from Japp-Maitland condensation through nucleophilic transfer.
The acetic anhydride in Perkin's reaction produces aromatic enolate.
In Dieckmann Condensation, 2 ester compounds are found in the same molecule, which would be important for the activation of cyclic compounds.
The Aldox process, introduced by Royal Dutch Shell and Exxon, transforms propene as well as syngas to 2-ethylhexanol using hydroformylation into butyraldehyde, aldol condensation to 2-ethylhexenal, and lastly hydrogenation.
Dehydration of Aldol Products
Dehydration of such primary Aldol products is possible, and it is frequently aided via these different parameters:
Alpha hydrogen should still exist between both the carbonyl and the -OH group within the Aldol molecule.
Examine for longer conjugated (i.e. enhanced stability) throughout the product.
Heating the process frequently promotes water removal.
Dehydration is preferred in non-aqueous reaction situations.
The procedure often called "Crossed Aldol Condensation," happens when two distinct molecules with carbonyl groups combine. That kind of reaction would be only feasible because neither of the compounds contains any alpha hydrogens. Just one nucleophile is created, and therefore only one enol/enolate is produced. Mixed reactions take place when a ketone, as well as an aldehyde with no alpha hydrogens, combines. As a result, the nucleophile is created totally from the ketone. In general, aldehydes are much more reactive to nucleophiles than ketones. It significantly decreases the potential of ketone self-condensation.
The most significant carbonyl compound reactions are aldol condensation (i.e. aldehydes and ketones). This process takes place when 2 molecules of aldehydes as well as ketones with at least 1 α-hydrogen atom condensate in the existence of dilute alkali and generate beta-hydroxy aldehyde or ketone. When aldol condensation products are heated with dilute acids, they dehydrate to create, α, β-unsaturated aldehydes and ketones. Because aldol products include substituent (-OH & –CHO), these may be employed in a range of reactions to produce a variety of products. Crossed aldol condensation is ineffective in labs when both carbonyl compounds contain alpha hydrogen,, so a wide range of products has been generated. Meanwhile, it is beneficial, when one of the carbonyl compounds lacks alpha hydrogen as well and hence, could perhaps initiate self-condensation.
What does aldol mean? Can you give a specific example of a reaction?
Ans: Aldols are Para hydroxy aldehydes and ketones formed either by condensation of 2 individuals of the same or one among 2 distinct aldehydes or ketones within the availability of such a dil. Aqueous base. For instance, Chemical Reactions of Aldehydes as well as Ketones-Oxidation.
In an aldol condensation, which catalyst has been used?
Ans: A novel manganese compound was built around an S,S-1,2-diaminocyclohexane connected ketopinic acid scaffold as well as effectively used as a catalyst throughout the aldol condensation reactions of benzaldehyde using different aliphatic ketones, yielding results with > 99% yield.
What exactly is the contrast between aldol reaction versus aldol condensation?
Ans: The Aldol Reaction occurs when a carbonyl molecule enolates using aldehydes as well as ketones to generate a beta-hydroxy carbonyl compound; whereas if circumstances result in further dehydration and form the α, β-unsaturated substance, the reaction is known as the Aldol Condensation.
