We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
That stuff we see every day is matter, a sort of substance. It may be physically grasped and feels substantial. The quality of the air we breathe and the water we drink is also important. Thus it's safe to say that matter is something we encounter every day. A single atom is the smallest unit of matter and the building block of all others. Elements and compounds are categorised by the atomic makeup of their constituents. In terms of atoms, there is just one kind present. Several atom kinds can be present in compounds. All three states of matter—solids, gases, and air—have useful properties. Many chemical and physical attributes need an understanding of the topic at hand.
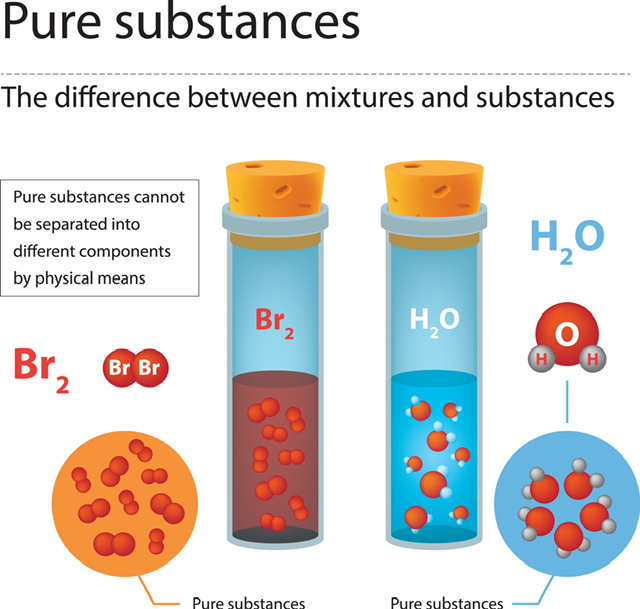
The term "matter" is used to describe any substance that is not empty, has mass, and occupies space. There are three distinct varieties, distinguished by their underlying physical states. They can be a gas, liquid, or solid. Matter may be divided into two categories, pure substances and mixtures, depending on its chemical condition. Hence, matter is not a single, unmixed entity, but rather a mixture of several. Pure matter consists of a single element or kind of material, while matter composed of two or more elements or types of substances is a mixture. In the form of mixtures, many different kinds of matter may be found. Everything we can see exists in a more complex, less pure form than its pure counterpart.
A mixture consists of two or more substances that do not chemically combine to form a single compound. Physical mixing is the only means for many substances to be mixed into solutions, suspensions, or colloids while still retaining their own identities. They can be physically split apart. To put it simply, there is no chemical chemistry involved in the production of the many parts that make up a given combination. Therefore, the individual characteristics of each constituent remain unchanged.
A mixture, on the other hand, is the result of combining two substances and subsequently separating them without any adverse chemical reaction. All individual chemicals in a mixture keep their individual identities. Mechanically blending the components or complex without any chemical bonding occurring results in a mixture, and it stands to reason that all elements involved in the mixing process retain their original chemical characteristics. Although mechanical mixing is common, mixtures can also be created through other means (e.g., diffusion, osmosis).
If the particles in a heterogeneous mixture are large enough, various physical approaches may be able to help the separation. Evaporation, centrifugation, chromatography, and other techniques can be utilized if the particle size is too fine to be separated by conventional methods. They constitute,
If the particles are in a combination or are volatile, you can get rid of them by evaporating them. Heating is a viable option for doing this.
To separate smaller particles from bigger ones, a centrifugation device can be used.
Sublimable components can be separated by the sublimation process.
Chromatography is a technique for separating mixtures based on the sizes and colours of their individual components. Even cutting-edge chromatography equipment may be found here.
Condensation is the process through which vaporized liquids are returned to their original form.
For two or more substances to be considered a solution, the combination must be uniform throughout. The solution consists of the two fundamental elements, the solute and the solvent. The solute dissolves in a solvent, which might be an abundant component or just another medium. The components of the solution are so little that they can only be found in the solvent. When a solute dissolves in a solvent, it can no longer be extracted using traditional physical methods. Particles cannot be detected by any means because of its high stability. Salt water, sugar water, and other similar mixtures are all examples of solutions. Several different solutions exist between different types of solids, liquids, gases, etc. The solid-liquid kind is the most common and well-known.
Solution
Physical and chemical changes are associated with matter. They are different terms associated with the change happening on a substance. Some of the differences between these two terms are tabulated below.
You can categorize pure substances into two categories. To put it simply, they are:
Matter is a material with a measurable mass that occupies its own space and is all around us. They play a crucial role in the analysis of all substances' physical and chemical properties. There are two primary types: pure and mixed. As opposed to a combination, which might include many substances, a pure substance only includes the one that it is named after. Much of the stuff around us is a composite of several substances. In addition, they might have a consistent makeup or have varying characteristics. Several techniques exist for separating heterogeneous mixtures. To be more precise, the answer is an example of a homogeneous mixture, in which the constituent elements are all equally distributed.
A true solution is one which is a homogeneous mixture. It consists of the same phase throughout with uniformity. The true solution has a particle size smaller than 1nm.
A suspension is a heterogeneous mixture. Its particle size is more than 100 nm. Its particles can be seen with the naked eye. Suspension shows the property of Brownian motion and the Tyndall effect.
Anything that has a physical existence in this world with proper physical dimensions, such as height, weight, and volume, is called matter.
