We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
When the bonding in a molecule or polyatomic ion cannot be represented by a single Lewis formula, the phenomenon of resonance is used to describe the behavior of the delocalized electrons involved. Several resonance structures can be used to depict a molecule or ion with delocalized electrons. The Lewis nuclear framework The only thing that changes about these resonance structures is the position of the electrons in them. They are extremely important in chemistry and aid in deciphering the structures of several compounds. These bonds are central to the valence bond theory, which attempts to provide a mechanistic account of the chemical interactions between different elements.
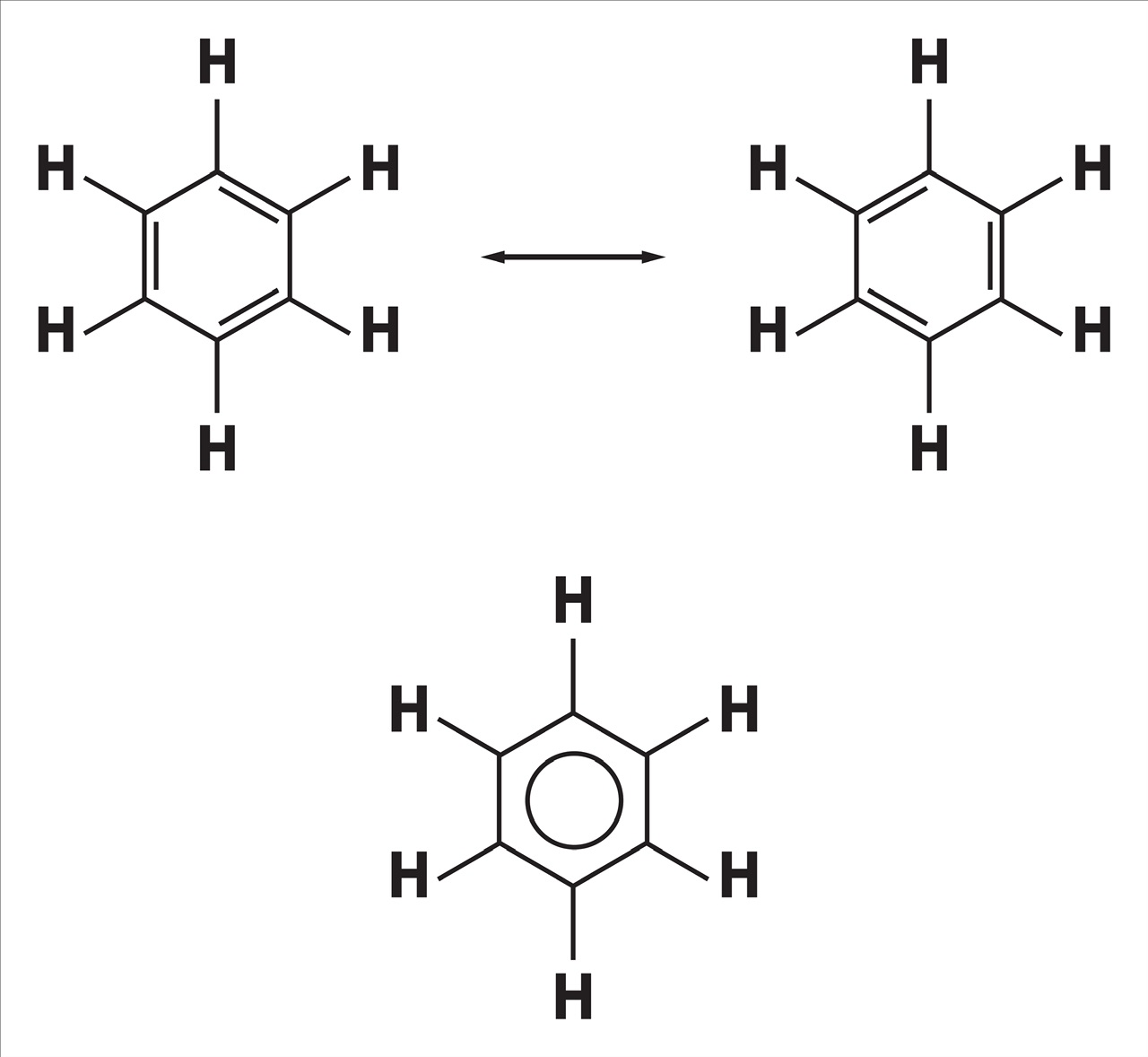
This idea was initially offered in 1899 by Johannes Thiele in his "Partial Valence Theory" to explain the unexpected stability of benzene, which followed August Kekulé's structure described in 1865, which had consecutive single and double bonds.
Unlike most alkenes, benzene goes through substitution reactions rather than addition reactions. He contended that benzene's carbon-carbon bond is a single and double-bond hybrid.
The resonance concept also partially explained a large number of benzene derivative isomers. If we apply Kekulé's structure to the molecule dibromo benzene, for instance, we get predictions for four different isomers comprising two ortho isomers in which the brominated carbon atoms are connected by either a single or double bond. In actuality, there are just three di-bromobenzene isomers, and only one of them is ortho, which is consistent with the notion that there is only one sort of carbon-carbon bond, which lies between a single and a double bond.
The double bonds in some compounds may be twisted or rearranged, known as resonance or mesomerism. The mesomerism increases the compound's stability and, thus, its reactivity. Covalent chemistry describes the bonds between some chemical compounds, such as benzene, ozone, etc. When the Lewis structure fails to express and explain delocalized electrons sufficiently, it also refers to a strategy for expressing and explaining them.
It occurs when a nonbonding electron is present or when a pi bond is altered. The pi-electron locations or even the positions of nonbonding electrons cause an atom's orbital to change. Each of the resonance structures must possess the same degree of energy. It happens in unsaturated systems due to the delocalization of electrons during this process.
Resonance energy is linked to the concept of aromaticity. Resonance energy is used as a first step in determining the stability of a resonating structure. Because the bonding in a molecule cannot be expressed uniquely by a single Lewis structure, the presence of resonance energy can be used to identify the delocalized electrons present in the molecule.
The resonance energy may be calculated by comparing the energies of two compounds, one with a localized Lewis structure and the other with a delocalized real structure.
A mathematical representation of resonance energy follows
∆E = Edelocalized - Elocalized.
The more the value of resonance energy, the more stable the compound.
One of the most important hydrocarbons for research in organic chemistry is benzene (C6H6). It has a cyclic structure with alternating single and double bonds. Benzene's ring structure allows for two distinct resonance modes, one of which involves delocalizing the pi-electrons.
The six carbon atoms that make up benzene are all sp2 hybridized, with an unhybridized p orbital running perpendicular to the ring plane. Because of the equivalence of the p orbitals of the 6 carbon atoms, it is inconceivable for them to overlap with only one neighboring p orbital to form three distinct double bonds. The six p orbitals instead overlap cyclically, each with its neighbor. This allows for greater overlap between the p orbitals than would be achieved from the linear 1,3,5-hexatriene analogue since the p orbitals are delocalized into molecular orbitals that stretch around the ring.
The ring must be planar for this to occur, because without it, the p orbitals would not be able to overlap appropriately, and benzene is typically thought of as a planar molecule.
The ozone (O3) molecule consists of a central oxygen atom singly bonded to one oxygen atom and doubly bonded to another. There is no net charge on this molecule, but the Lewis structures of this molecule show a +1 charge on the central oxygen and a -1 charge on the singly bonded oxygen.
The molecular structure of ozone predicts a single O-O bond and a double bond O=O. Different bond types (one single and one double, for example) would result in varied bond lengths, but it was determined that the O-O bonds are the same: 127.2 pm, shorter than a normal single bond and longer than a double bond.
Carbonate ion is greatly useful in the chemical field and widely used for cooking. It is a polyatomic ion generally found in baking powder or baking soda. During cooking, it releases carbon dioxide, making the food contents aerated, fluffy, and soft. This technique is used for making cakes, bread, etc. As carbonate ions come into contact with acid, carbonic acid is produced, a crucial ingredient in aerated drinks.
2 out of 24 electrons of the Carbonate ion have the -2 charge. The carbon atoms are required to share electrons to satisfy the octet rule and thus, attain a certain level of stability. Thus, the reconfiguration of electrons takes place to make double bonds with the carbon molecule. The valence gets filled, and the positive charge on the carbon atom gets canceled.
Carbonate ion
The most important features of resonance in chemistry are:
Understanding the chemical structures of radicals, compounds, ions, etc. is crucial in organic chemistry, and one of the most important phenomena in this field is resonance.
By shifting the orientation of double bonds, makes the chemical structures of many compounds more stable. Benzene, nitrobenzene, and other molecules and compounds display this property.
It's useful for understanding why some chemicals respond the way they do. A compound's stability indicates how reactive it is in any chemical reaction. There is an inversely proportional relationship between these two factors.
In organic chemistry, resonance plays a crucial role. Resonance hybrids are molecules whose electron patterns defy description by a single Lewis structure. It is useful for learning about the chemical reactions that can occur in compounds that use covalent bonds. In 1899, Johannes Thiele proposed this theory in the "Partial Valence Theory" to describe the surprising stability of benzene, which resembled August Kekulé's structure published in 1865 and had sequential single and double bonds.
1. Is the Huckel rule valid for all aromatic compounds?
For a compound to be termed aromatic, it must be planar, cyclic, and conjugated and fulfill Huckel's rule. Huckel's rule asserts that an aromatic molecule must contain pi electrons in the overlapping p orbitals to be aromatic.
2. For what reason is aromatic more stable than antiaromatic?
A cyclic molecule that contains 4n delocalized ( or lone pair) electrons is considered antiaromatic because its electron system is more energetic than an aromatic molecule.
3. Can you explain why aromatics have a higher acidity level?
The acidity is reduced when the anion is destabilized by the electron-donating resonance and +I effect. There is a greater acidity in the chemical that produces aromatic anions than the compound that produces anti-aromatic anions.
