We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
A "mixture" consists of two or more components in a homogenous or heterogenous phase. There is no chemical link between the components of these mixes thus they can be separated by physical methods. "hand-picking, winnowing, filtering, distillation, etc." are only some of the methods used to separate them. Any two different types of matter can combine to form a new substance.
While attempting to separate a combination, one can choose from a number of different approaches.
Around 1840, some prospectors used water to extract gold from a mud combination. A pan containing the mud mixture and the gold was then dipped into the water.
Gold, being heavier than the dissolved material, dropped to the bottom of the pan after being agitated for a while. For those interested, "Panning" refers to the process of extracting gold from water.
Homogeneous and heterogeneous mixes are the two types of mixtures that naturally exist in our environment.
Different methods are utilized to break them apart.
There are various methods of separating any mixtures depending upon the type of mixture. In 1840, some prospects separated the gold from a mixture of mud by using water, the mud containing gold is filled with water in a pan.
After some time the pan was twisted to remove dissolved material and gold settled in the pan due to heavyweight. This method of separating gold from water is known as “Panning”.
In our environment, substances occur in the form of a mixture and there are two types, “homogenous and heterogenous mixtures”.
To separate them, different techniques are used.
Small-batch, large-particle mixes are separated by hand because of the advantages of this procedure. Its primary function is to remove unwanted stones from grains, rice pulses, and other foodstuffs throughout the preparation and sale process. Both parts of this kind are solid and large enough to be separated by hand.
It is usual practise to use this method to separate grains from twigs. Farmers employ this technique to separate the hard grains (such as wheat, rice, legumes, etc.) from their stalks. Food grains are gathered from the field and dried when they reach maturity.
The term "winnowing" refers to the process of using wind to blow away the husk from grains or pulses. A winnowing basket is used to collect the grains that still have their husks on them. The farmer raises his basket to a specific height and faces the wind.
When the farmer lets the grain fall into the wind, the husk and twigs are blown away, and the grain is cleansed.
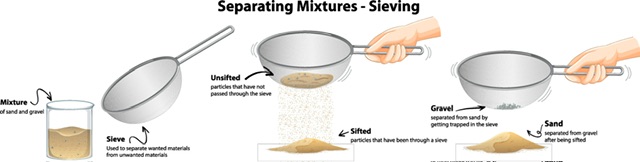
A sieve is a type of container with a fine-mesh iron net at the base. The process of sieving allows us to separate a solid mixture in which the particles vary in size. When doing this, the mixture is placed in a sieve and rocked back and forth.
The sieve allows the smaller particles to flow through while the larger ones are trapped.
After grinding, wheat flour is sieved to remove any unground bits.
The process of "evaporation" is used to convert the solid components of the combination into a liquid state. Once the volatile component evaporates, a solid of non-volatile composition is left in the container.
A liquid component of the mixture is evaporated at a high enough temperature in this technique. The fluid has separated, leaving the solids behind in the container.
This process is used to extract salt from saltwater.
Distillation is a process used to separate liquids that have been combined.
Plant with a flask, thermometer, condenser, and collecting flask constitute the "distillation apparatus" in this procedure (distillate). The distillation flask is heated to a certain temperature, where the mixture is distilled.
The liquid begins to boil and change into a vapour. A condenser is used to capture the liquid distillate from this vapour.
Title: Distillation Apparatus
It is a typical technique for removing solids from liquids. Filter paper is a porous substance used in the filtering process to remove liquid from a combination that contains particles big enough to clog the pores of the paper.
A separating funnel is used to separate components based on their densities into two immiscible liquid phases. One is an aqueous phase, and the other is an organic solvent.
Magnetic Separation
A mixture of magnetic and non-magnetic materials is separated by this method. The mixture is rolled over a magnetic wheel that separates iron and other non-magnetic material.
These environmental parts are typically found in sets with other parts. The mixes here are both homogeneous and heterogeneous. Any of the three states—liquid, solid, or gas—can describe the combination. There is no chemical link between the two materials. Depending on the nature of the combination, several techniques are used to separate the components, such as sieving, hand-picking, winnowing, and magnetic separation.
Extraction is a process of separating components of a mixture by dissolving one component in a solvent and then separating the components from each other. It is based on the solubility of the substance of interest. Polar compound dissolves in polar solvent and nonpolar compound dissolves in nonpolar solvent.
The difference between physical and chemical separation is that physical separation is a process of separating components of a mixture through physical means, while chemical separation is a process of separating components of a mixture through chemical means.
The advantages of chromatography include its ability to separate complex mixtures, its accuracy and precision, its speed, and its ability to detect trace amounts of substances.
