We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
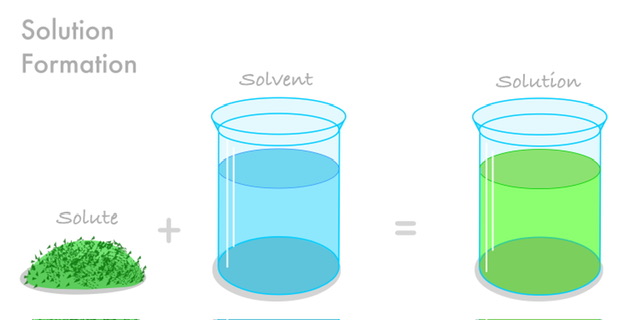
Whenever you want to mix any number of substances together, you need to create a solution, which is a completely uniform combination. All of the components of a mixture are the same in a homogenous one.
The true solution is the homogenous solution with each dissolved particle having exactly the same size as a molecule. Hence, molecular solutions are also used to describe true solutions. Almost everything you use on a regular basis—soda, deodorant, sugar, salt milk, tea, soda, etc.—contains solutions, therefore they are an integral part of your life. A solution is made up of two components: solute and solvent.
Solution
Substances that are dissolved in a solution are called solutes. The most abundant substance in a mixture or solution. Dissolving takes place between two substances; the solute and the solvent. Solute molecules are free to roam wherever in the solution. A solution is formed when molecules of a solute and molecules of a solvent combine through molecular interaction. Solvent molecules surround the solute molecules and can flow freely in and out of the space between them.
A solute's ability to dissolve in a solvent depends on the solvent's chemical makeup. As the characteristics of each solvent are unique, some solutes will dissolve better in some solvents than others. Sugar, for one, dissolves far more easily in water than it does in oil.
A solution's concentration is equal to the mass of the solute per unit volume of solvent. More solute dissolved in a given amount of solvent results in a greater solute concentration.
Some of the solution's characteristics, such its viscosity and surface tension, may change as a result of solvation. It is possible for solute molecules to interact with one other and with other solutes in a solution.
The process by which the solute molecules create bonds with other molecules in solution is called complexation. The solution's pH and electrical conductivity, for example, may change as a result.
It is also possible for solute molecules to interact with the solution's container walls. They can get adsorbed on the walls or undergo ion exchange. This takes place when the solute molecules interact with one another or with the container's walls and release or absorb ions. The solution's pH and electrical conductivity could change as a result of this.
A substance with the ability to dissolve another material (the solute) to create a uniform mixture is called a solvent. Many tasks need the use of solvents, such as cleaning, degreasing, and as a medium for chemical reactions.
In general, solvents are categorized as either polar or nonpolar. Molecules in polar liquids have a net dipole moment, indicating that there is a tiny positive charge imbalance between opposite ends of the molecule. Yet, the molecules in nonpolar liquids have no net dipole moment. In general, polar solvents are more effective at dissolving polar solutes, while non-polar solvents are better at dissolving non-polar solutes.
Solubilizing substances are often dissolved in water, alcohols, or organic solvents like acetone, benzene, or toluene. As the name implies, water can dissolve just about anything. Alcohols are another common solvent, and they are frequently employed in degreasing and cleaning tasks. As organic solvents can dissolve a large variety of organic molecules, they are frequently utilized in chemical processes.
Similarly, solvents can be divided into two groups: volatile and non-volatile. In contrast to non-volatile solvents, which remain in liquid form even after being exposed to air, volatile solvents soon evaporate. As volatile solvents evaporate quickly and leave a little trace after being used for cleaning, they are frequently put to use for this purpose. Non-volatile solvents are useful in chemical reactions because they can be simply poured off when the reaction is complete.
Some of the properties of a solution are:
A solution is a combination of two or more components that is completely homogenous.
The particles that make up a solution are sufficiently tiny to continue to float freely in the solvent.
The solute particles have size smaller than 1nm.
With unaided vision, one cannot make out the individual components that make up the solution.
The particles that make up a solution will not get separated from the solution as time passes.
Solutions can be categorized in a variety of ways, some of which are:
Water-based: It's possible to divide solutions into two categories: those containing water and those that don't.
Aqueous Solution
The condition of a substance in which it entirely dissolves in water is called a solvent solution. Sugar/salt in water, and carbon dioxide in water, are both examples of solutions.
Organic solution
These solutions are diametrically opposed to aqueous ones because the solvent at hand is not water but something else entirely, such as gasoline, benzene, ether, etc. Phenolphthalein in benzene, sulfur in carbon disulfide, etc., are examples of such solutions.
Solute Concentration: Depending on the concentration of the solute, there are three distinct types of solutions.
Saturated solution
When no additional solute can be dissolved in a solvent at a given temperature, the solution is said to be saturated.
Unsaturated Solution
The solution is considered unsaturated if it can dissolve more solute in a solvent.
Supersaturated solution
Supersaturated solutions are those in which there is an excess of solute, which may be forcibly dissolved in the solvent by increasing the temperature. Crystals are formed from these surplus solute particles by a process called crystallization.
A homogeneous combination of two or more components is called a solution. A solution is made of two components: a solute and a solvent. Both the solvent and the solute in a solution can be in any phase of matter (solid, liquid, or gas). Hence, there are nine distinct classes of solutions, each characterized by a unique combination of solute and solvent properties. Solutions are in everything you use, including soda, deodorant, sugar, salt, etc.
Temperature and pressure conditions affect the solubility. In liquids, solubility increases with temperature while in gases it decreases. Change in pressure however, has no effect on solubility in liquids but it increases in gases.
A solution that has a low boiling point and high vapor pressure generally forms a volatile solution. They tend to evaporate easily at room temperature. For example, methanol, acetone, etc.
The Tyndall effect describes the scattering of light by colloidal or ultrafine suspension particles. Blue light is scattered more strongly than red light; this phenomenon, called Tyndall scattering, is analogous to Rayleigh scattering because the intensity of the light scattered is inversely related to wavelength.
