We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
Everything which has mass and uses space is considered to matter. The cosmos is made up of this substance, which may exist in one of four unique states: solid, liquid, gas, or plasma. To have a complete comprehension of the physical universe, one must have a firm grasp of the many qualities and behaviors that are associated with the various states of matter.
Understanding the physical universe requires having a firm grasp of the four different states that matter may exist in. To fully comprehend the cosmos, one must first have a solid foundational knowledge of the many states, each of which possesses a distinct set of characteristics and actions.
Different states of matter can be defined by the degree to which its defining qualities shift in response to environmental factors like pressure and temperature. When the temperature of ice is raised above 0 degrees Celsius (32 degrees Fahrenheit), a discontinuity occurs because energy is diverted from the temperature increase to the phase transition.
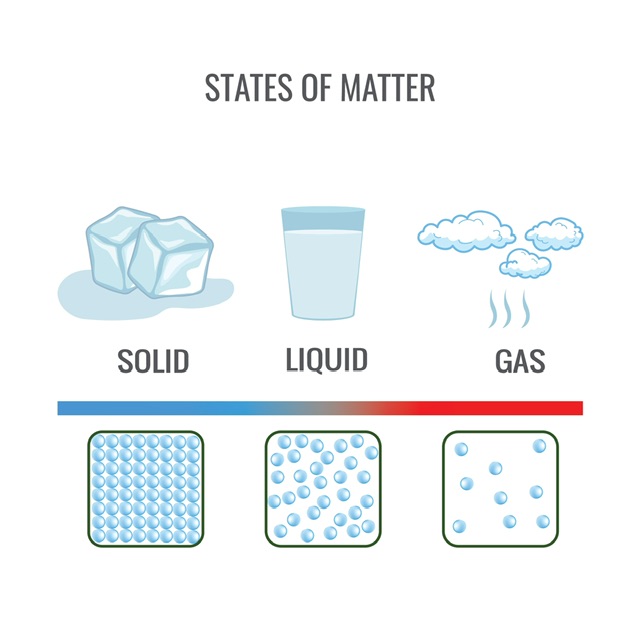
It is said that atoms, molecules, and ions are in a solid state when their structures are hard and organised. The particles in this condition are held together by strong intermolecular forces, preventing them from moving freely. Materials in the solid state tend to be tough and unyielding; they may also have a crystalline or amorphous structure.
Some properties of the solid state are:
To a large extent, the properties of solid state materials are dictated by the nature of the bonds between their constituent particles. The particles of crystalline materials are organized into a predictable, repeating pattern and are bound together by strong bonds. It's because of this that the material is so sturdy and rigid. The particles of inorganic materials are not consistently arranged, and the connections between them are weak and pliable. Because of this, the material may be shaped and bent without breaking.
The strength of solid state substances is a crucial characteristic. Materials in the solid state are often exceedingly sturdy and resistant to damage under high stresses.
The ability to conduct electricity is a crucial quality in solid state materials. A wide variety of solids are excellent conductors of electricity. Because of this, they find usage in the construction of electronic circuits and other electrical engineering components.
The optical characteristics of solid phase materials also vary widely. Certain solid state materials are see-through because they don't block the passage of light. They can be utilized as mirrors and lenses because of their property. Opaque substances are those that prevent light from penetrating the material. This makes them practical for use as glass windows and other types of barriers.
Last but not least, the thermal characteristics of solid state materials can range widely. A lot of solid state materials are effective thermal insulators, meaning they prevent heat transfer.
The second type of stuff that may exist is liquid. Their fluidity and ability to flow freely are defining characteristics of them. In contrast to solids, liquids are made up of particles that are only haphazardly packed together and are only kept in place by intermolecular interactions that are much weaker. Since these forces permit the particles to move about, liquids are able to flow and adopt the shape of the container in which they are contained. Water, oil, and alcohol are all types of liquids that may be found in the world.
Some of the properties of liquids are:
One of liquids' most notable characteristics is their ability to conform to the shape of its container. It's because liquids' molecules aren't packed as closely together as solids that allow them to flow. This gives them more freedom to roam their confines.
Liquids also have a defined volume, which is a useful attribute. This indicates that the volume occupied by a fixed volume of liquid is independent of the geometry of the container. This is because the molecules in a liquid are prevented from expanding or contracting by intermolecular interactions.
Similarly to gases, liquids may move about. The reason for this is because liquid molecules are not packed as densely as solid molecules. This gives them more freedom to roam their confines. Liquids can be used as a coolant in HVAC systems or as a cleaning agent because of this feature.
A liquid's viscosity is low because of its state. This implies they don't have the same resistance to flow as solids and may be manipulated with relative ease. Thanks to their quality, they find widespread usage as lubricants and hydraulic fluids, among other things.
Gases are the third state of matter after solids and liquids. Its low density and ability to expand and fill any container distinguish them from other similar substances.
Gases are made up of particles that are extremely far away from one another and are only kept in place by intermolecular interactions that are very weak. Since these forces permit the particles to move about freely, gases are able to expand and fill any container that they are placed in. The elements air, oxygen, and helium are all examples of gases.
Some of the properties of liquids are:
Pressure from gases may be felt in every direction . The particles are smashing into the inside of the container, causing the pressure.
Gas particles contain enormous intermolecular gaps. Most of this gap may be reduced by the application of pressure, bringing the particles closer together. This allows for a significant decrease in gas volume. We call this process "gas compression."
The volume of a gas can be reduced by cooling it. If you lower the temperature, the particles have less energy, become less mobile, and drift farther apart from one another. Hence, the intermolecular attraction strengthens and the particles approach one another. The volume of the gas is therefore reduced.
A decrease in volume results from increased pressure on a gas. Yet, when pressure is released, gas expansion occurs.
Increasing the temperature causes the particles to become more energetic, accelerate, and spread apart. As a result, there is less of an emphasis on the intermolecular pull. The amount of gas expands.
Each and every one of the gas's molecules is constantly racing along at an incredible rate. The space between molecules, known as intermolecular space, is extremely large. When two gases are combined, the intermolecular space between their molecules becomes permeable to particles of the other gas. The result is a uniform and full blending of the two gases. Hence, a gaseous mixture retains its consistency at all times.
Gases have enormous volumes in comparison to their masses because of the huge intermolecular gaps between their molecules. Because of this, their densities are lower.
Title: Compression and expansion of gas
The fourth and last state of matter to be described is plasma. Both its great energy and its capacity to carry electricity are defining characteristics of it. Ionized particles are what make up plasma, which is kept together by powerful electromagnetic forces. Plasma is made up of these particles. These forces enable the ions to freely move, hence plasma conducts electricity. Lightning, the sun, and the stars are all forms of plasma that may be found in nature.
The article explains the differences between the solid, liquid, and gaseous phases of matter. Although solids maintain their original shape and volume when placed in a container, liquids conform to the shape of the container, and gases completely occupy the space available. The article describes the three states as well as how temperature and pressure impact them. It also clarifies the transition between states, or phasing, and how it occurs. The article concludes with discussing plasma, the fourth state of matter, which is composed of charged particles and may be found in galaxies.
A gas of very low-density bosons can condense into a Bose-Einstein condensate if chilled to temperatures near absolute zero, making it the 5th state of matter in condensed matter physics. Gases like BECs aren't found on Earth, but they may form in the high pressure environments around neutron stars.
In a gas, the atoms are whole and usually bound together to form molecules, but in a plasma, at least part of the electrons have been completely decoupled from their atomic nuclei. In other words, gas particles are typically neutral whereas plasma particles have electric charges.
So far as science is aware, no metals have been discovered to have a gaseous phase at ambient temperatures. With the exception of mercury, which is a liquid at normal temperature, all metals are solids.
