We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
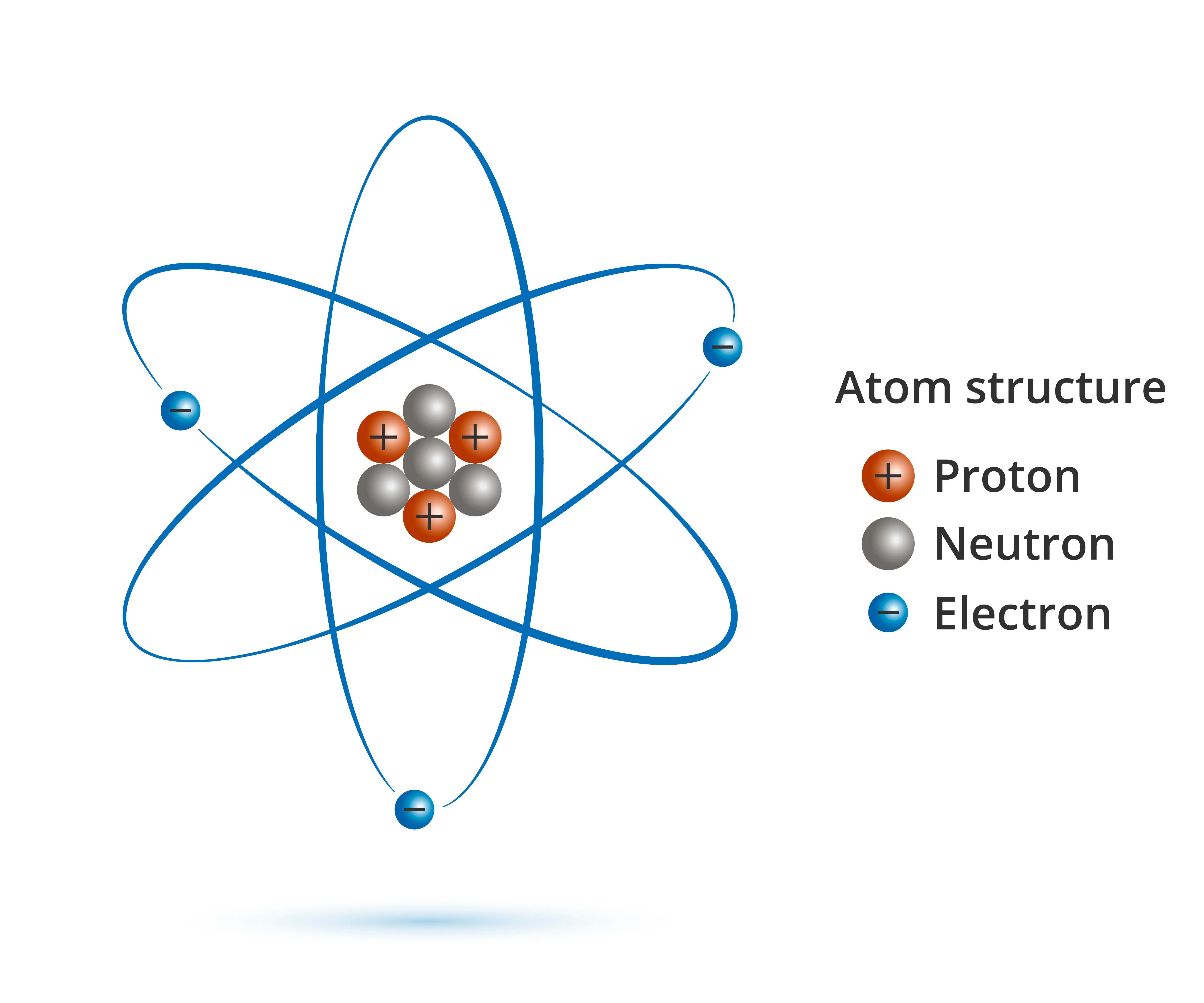
All stuff is composed of smaller and smaller units called atoms. Neutrons, electrons, and protons make up the atomic nucleus. The nucleus of an atom is composed of protons and neutrons, and the electrons move in circular orbits around the nucleus. Atoms can join together in chemical bonds through the exchange of electrons. The molecular structure is produced in this way. The quantity of electrons exchanged or shared determines the sort of bond created. When two atoms share electrons, a covalent link is established; when one atom transfers electrons to another, an ionic bond is created.
Atom
The bulk of matter is made up of molecules that can be easily isolated. Yet, molecules are made up of atoms held together by chemical bonds which are far more robust. At its most basic level, an atom is made up of a nucleus and an electron cloud. The electric forces exerted by the charge on these particles are what keep the atom together. Separation efforts for these subatomic particles use ever-increasing quantities of energy and produce new subatomic particles, which are frequently charged.
As was said at the outset of this piece, an atom is mostly made up of nothing but empty space. The nucleus, located in the center of an atom, is positively charged and holds the vast majority of the atom's mass. It is made up of positively charged protons and neutral neutrons. In all naturally occurring atoms, you'll find long-lived particles like protons, neutrons, and the electrons that orbit them. The presence of all these 3 kinds of particles may be accompanied by the presence of other subatomic particles. Yet, they need a great deal of energy to produce and have a relatively little lifespan.
The number of positive charge units (protons) in the nucleus is the atomic number (typically represented by the symbol Z), which is the most significant property of an atom. A Z value of 6 indicates carbon, whereas a Z value of 92 indicates uranium. When the ratio of protons and electrons in an atom is balanced, the resulting atom is electrically neutral. A nucleus's number of protons is the ultimate determinant of an atom's chemical characteristics, as the electrons are responsible for establishing the atom's interactions with other atoms.
The mass of an atom is proportional to the number of protons, but not to the number of neutrons in its nucleus. Chemical characteristics are independent of nucleon number; a nucleus with 6 protons and 6 neutrons will have the same properties as a nucleus with 6 protons and 8 neutrons, despite their differing masses. Isotopes are atomic nuclei that share the same number of protons but have differing neutron counts. Each chemical element has several isotopes.
The atomic mass number, which is the sum of the nucleus's protons and neutrons, is commonly used to describe various isotopes. Considering that the first carbon atom has 12 protons and 6 neutrons, it is designated as 12C, whereas the second is 14C.
Since the late 19th century, researchers have understood that the electron has a negative electric charge. In 1909 and 1910, American physicist Robert Millikan performed the first measurements of this charge's worth. Millikan performed the oil-drop experiment by suspending small drops of oil in an oil mist within a sealed container. It was the pace of descent that allowed him to calculate the oil drips' density.
When an electric force was applied to oil drops that already carried an electrical charge (from, say, air friction), the drops' forward motion may be delayed or stopped entirely. Millikan found the electric charge on each drop by comparing the amount of applied force to the resulting motion variations. He measured a large number of drops and discovered that their charges were all simple divisors of the same number. The charge on an electron served as the standard measuring stick, with each oil drop representing 2, 3, 4, etc., more electrons. Now, scientists generally agree that an electron has a charge of 1.602176565 × 10-19 coulombs. In 1923, Millikan was honored with the Nobel Prize in Physics for his contributions to this field.
One of the fundamental constituents of matter is the subatomic particle known as the proton. A proton is a charged particle that can be seen in the atom's center. Protons represent the most common subatomic particle and play a crucial role in the building of the molecules and atoms in the universe. A proton consists of three quarks: two up quarks and one down quark. The strong nuclear force is the greatest force in the universe and is responsible for keeping the quarks together. Around 1,836 times the mass of an electron, the proton has a mass of 1.6726 x 10-27 kg,
The nucleus of an atom may be compared to the sun, and the electrons can be compared to the planets in a small solar system. The amount of protons in the nucleus establishes the energy levels that the electrons occupy as they orbit the centre of the atom. The chemical characteristics of an atom are determined by the valence electrons, which occupy the atom's highest energy level.
Protons, neutrons, and electrons make up the tiniest atomic particles possible. Knowledge of atomic structure is fundamental to comprehending the nature of matter and its interactions with other particles.
A chemical bond is an attractive force between two atoms or molecules that allows them to form a chemical compound. The types of chemical bonds include covalent bonds, ionic bonds, and hydrogen bonds.
Democritus, a Greek philosopher, proposed the concept of the atom around 450 B.C.
Despite this, for almost two thousand years nobody paid much attention to the concept at all. John Dalton re-introduced the atom in 1800. He established the existence of atoms and pioneered the field of atomic theory.
Isotopes are the extended families of elements. In an isotope, the number of neutrons can vary, but the number of protons remains constant among all members of the element's family. In the Periodic Table, an element's atomic number corresponds to its number of protons.
