We'll be back in a bit !
The system is currently undergoing a routine upgrade to ensure you get the best learning experience. The maintenance is expected to continue till 8:00 pm. Please check back later.
Thank you for your understanding!
Everything in this universe is made up of very tiny entities known as molecules. Based on the distance between these molecules, the matter is classified into solids, liquids and gasses and these different states of matter have different properties. Common examples include temperature, density, pressure, etc.
Heat in the universe around us is transferred via three processes, which are named conduction, convection, and radiation. The study of energy that stems from heat is done under a branch of physics known as thermodynamics and this branch discusses everything from heat, work, temperature, to their relationship with energy, entropy, and various other physical quantities.
Heat is a form of energy and it is transferred across different bodies when they carry a temperature difference. Heat energy is related to the speed of vibration of the molecules of an object. The hotter a body is, the faster its atoms and molecules vibrate and the more energy it carries.
Heat is always transferred from a hotter to a colder body and it can convert solids into liquids via melting. It can also cause evaporation, which is the conversion of liquids into vapor form.
Unit of heat
As a form of energy, heat is measured in units of Joules where one Joule is defined as the amount of heat energy required to change the temperature of a body by one unit.
Another commonly used unit of heat is known as the calorie, which is the amount of energy required to change the temperature of a gram of water by 1 unit. This is equal to 4.184 Joules.
Other commonly used units include the British Thermal Unit, which is defined as the amount of heat energy required to change the temperature of a pound of water by one unit.
It is easy to convert across different units of heat and one just needs to keep the conversion factors in mind. We list a few common conversion formulae below:
Conversion of BTU into Joule and Calorie
The following equation can aid us in converting BTU to Joules.
1 BTU=1055.06 J
And since one calories equals 4.184 Joules, we have
1 BTU=251.99576 cal
Conversion of Calories into Joule
As previously mentioned, we can use the conversion factor of 4.184 for converting between calories and joules. That is,
1 Calorie=4.184 J
The concepts of heat have been studied for a long time, but the most complete theory was first proposed in 1798 by Joule, who published a paper titled “Mechanical Equivalent of Heat”. This paper discussed concepts which allowed us to relate heat with mechanical work. Further, studies on thermodynamics evolved during 1850-1860 and the kinetic theory of gasses was introduced in 1927.
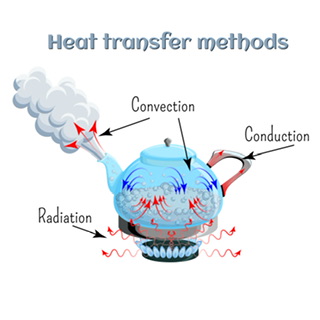
Heat can be transferred across different bodies in the following ways:
Conduction: Conduction is the transfer of heat energy between molecules of a substance due to collisions between its molecules. A hot body has molecules that are rapidly vibrating about their position. These vibrating molecules impart their energy to nearby molecules, which start to vibrate. This process continues till heat energy is transferred across the whole conductor. Note that the molecules only vibrate about their mean positions but do not travel across the conductor. Generally, solids make the best conductors.
Transfer of heat
Convection: Convection involves heat transfer via the actual movement of hot molecules of a substance. Liquids and gasses undergo this process since their molecules can move from place to place. The hotter molecules rise towards the top while the colder ones tend to sit down, causing heat transfer.
Radiation: Radiation involves heat transfer without any medium. It occurs via the emission and absorption of electromagnetic waves, which are emitted from hot bodies. Generally, hot bodies emit EM waves in the infrared region but very hot bodies can emit in the visible or even ultraviolet region.
1. Determine the amount of heat energy dissipated if 50 kg of water is cooled down from 800 ℃ to 500 ℃. The specific heat of water is
We are given that. The relation between heat and specific heat capacity is given by Q=mcT. In this example, the change in temperature, ΔT=(800℃-500℃)= -300 ℃, where the negative sign signifies heat loss. Hence,
2. How much heat energy is required to raise the temperature of 1 kg of iron from 150℃ to 500 ℃. The specific heat of iron is Q=mcΔT
In this article, we developed an understanding of the concept of heat, its units, and conversion across units. Further, we analyzed how to solve numerical related to heat transfer using a few solved examples.
1. What are the uses of heat energy?
Heat is an inseparable part of our lives and we list a few applications below:
Thermal power plants utilize heat energy to generate electricity.
Heat therapy is used to aid in pain relief.
Automobiles make use of heat energy generated by burning petrol and diesel.
We cook our food using heat.
2. How are heat and temperature related to each other?
Temperature and heat are closely related. Temperature measures the average kinetic energy of molecules. And heat is the form of energy that gives rise to the vibration of molecules.
3. What is a calorimeter?
A calorimeter is used to measure how much energy is involved in a chemical reaction. It is based on the principle of conservation of energy.
4. What is the law of conservation of energy?
The law of conservation of energy states that energy in this universe can never appear or disappear out of nowhere. It can only be transferred across its different forms. It is also possible to create energy from the mass using Einstein’s mass-energy equivalence.
5. What is specific heat?
Specific heat of a substance refers to the amount of heat energy required to raise the temperature of one unit mass of that substance by one unit.
